- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
 Scroll DownPopular Treatments
Scroll DownPopular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Topical Finasteride Calculator
- Interactive Guide: What Causes Hair Loss?
- Free Guide: Standardized Scalp Massages
- 7-Day Hair Loss Email Course
- Ingredients Database
- Interactive Guide: Hair Loss Disorders
- Treatment Guides
- Product Lab Tests: Purity & Potency
- Evidence Quality Masterclass
More
Articles100+ free articles.
-
Cannabidiol (CBD) Increases Hair Counts By 246%? Not So Fast.
-
Creatine: Does It Worsen Hair Loss? It Depends On The Hair Loss Type.
-
Can Progesterone Improve Hair Regrowth?
-
CRABP2: Can This Gene Predict Regrowth From Retinoids?
-
BTD: Can This Gene Predict Regrowth From Biotin?
-
COL1A1: Can This Gene Predict Regrowth From Collagen Support?
-
2dDR For Hair Loss: What Do We Know So Far About This Sugar?
-
CYP19A1: Can This Gene Predict Regrowth From Hormone Therapy?
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
ArticlesHMI-115: What We Know So Far
First Published May 9 2024Last Updated Oct 23 2024Company ReviewsPharmaceutical Researched & Written By:Sarah King, PhD
Researched & Written By:Sarah King, PhD Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor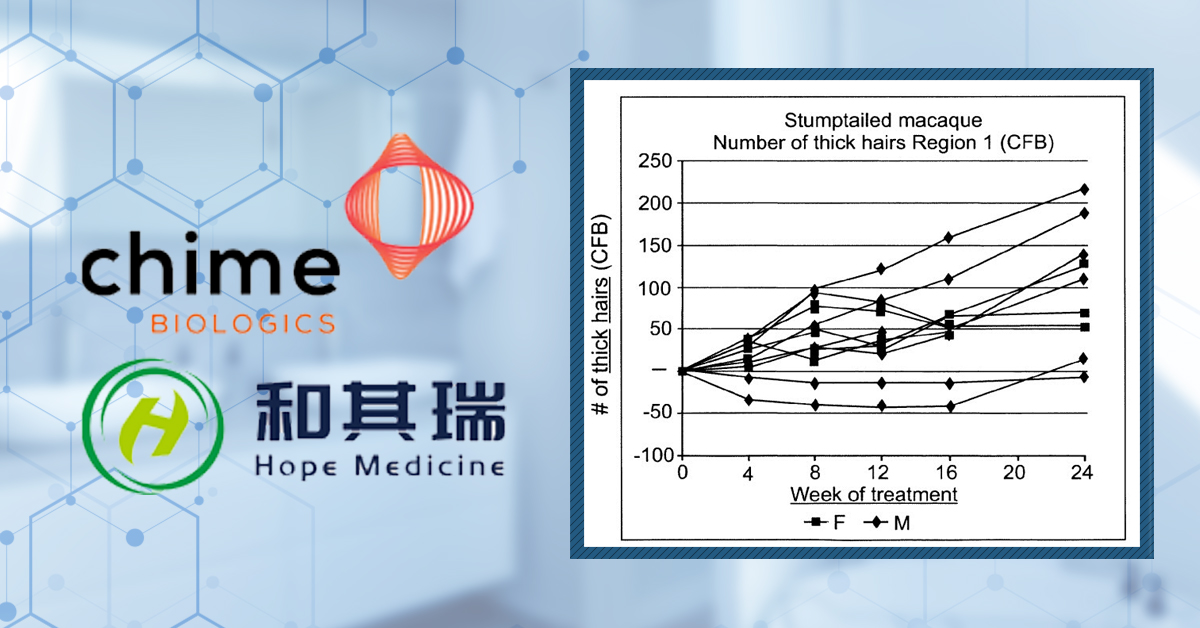
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
HMI-115 lowers prolactin levels and is currently in clinical trials for the treatment of androgenic alopecia (AGA). Internal, non-peer-reviewed research suggests that HMI-115 may promote hair regrowth in monkeys. Anecdotes from its phase I trials in humans imply that HMI-115 may also regrow hair in men with AGA. But is this the whole story? In this article, we’ll review the totality of evidence on HMI-115 – its novel mechanisms, safety, & efficacy – and explore the nuanced data on prolactin levels and pattern hair loss.
Full Article
HMI-115 is a potential new treatment for hair loss that is licensed by Hope Medicine Inc. HMI-115 has been used in a phase 1 clinical trial for hair loss and has been approved by the FDA for phase 2 trials. This treatment is also in phase 2 clinical trials for use in endometriosis. HMI-115 is gathering media attention as a treatment that may come to the market within the next few years.
HMI-115 belongs to a class of drugs known as prolactin (PRL) receptor antagonists – it works by blocking the effects of prolactin within the hair follicle by binding to and blocking the prolactin receptor. In this article, we will take a look at what HMI-115 and prolactin are and then consider how prolactin might be involved in hair follicle biology and hair loss disorders. Furthermore, we will investigate the potential impact of HMI-115 in treating androgenetic alopecia and determine whether it is likely that phase 2 clinical trials for HMI-115 will return positive results.
Key Takeaways
- Drug. HMI-115 is a drug that comes under a class of therapeutics called prolactin receptor antagonists. This drug is licensed by Bayer, which is investigating its use as a drug treatment for endometriosis. HMI-115 is also licensed by Hope Medicine Inc., which used the drug in a phase 1 clinical trial for patients with androgenetic alopecia. The FDA has also approved HMI-115 for phase 2 trials for treating hair loss, although these trials have not yet started.
- Clinical Data. In a press release, Hope Medicine Inc. reported results from its phase 1 trial, which included a significant increase in the mean non-vellus total area hair count across the 12 males in the trial—an increase of 14 hairs/cm2 after 24 weeks. The study has not been peer-reviewed, nor have the full results been published. Furthermore, no data was provided for the 4 women included in the study.
- Safety. There was a phase 1 safety trial completed by Bayer (who licensed HMI-115 to Hope Medicine Inc.) in which up to 90 mg of the drug, delivered via subcutaneous injections every 2 weeks, was found to be well tolerated. This study, however, aimed toward using HMI-115 to treat endometriosis. The phase 1 clinical trial on hair loss, sponsored by Hope Medicine Inc., delivered 240mg of HMI-115 via subcutaneous injection every two weeks; they reported it to be safe and tolerable, though no supporting data was provided.
- Evidence Quality. HMI-115 serum scored 21/100 for evidence quality, by our metrics (this is subject to change depending on the results of clinical trials currently being undertaken).
What is HMI-115?
HMI-115 (also known as BAY1158061) is a new pharmaceutical drug developed by a biopharmaceutical company called Bioinvent and licensed by Bayer – a German multinational pharmaceutical and biotechnology company. HMI-115 has been subsequently licensed from Bayer by Hope Medicine Inc. for use as a hair loss treatment. So far, research around HMI-115 is geared toward treating endometriosis and male and female pattern hair loss. In terms of administration, HMI-115 is delivered via subcutaneous injection.
HMI-115 is a prolactin receptor antagonistic antibody.[1]Hope Medicine, (no date). Clinical Development. Research and Development. Available at: https://www.hopemedinc.com/research-platform?tp2 (Accessed: 21 April 2023) This is a type of therapeutic antibody that binds to the prolactin receptor, thereby inhibiting its activity by stopping prolactin from binding to its receptor.[2]Ferraris, J., Bernichten, S., Pisera, D., Goffin, V. (2013). Use of prolactin receptor antagonists to better understand prolactin regulation of pituitary homeostasis. Neuroendocrinology. 98. 171-179. … Continue reading Prolactin is a type of hormone produced by the pituitary gland. As indicated by its name, prolactin is a hormone that promotes lactation (milk production). In reality, prolactin is a pleiotropic hormone with many different functions in both men and women. We will go into more detail below about prolactin, its potential role in hair loss, and how HMI-115 might work to restore hair growth by inhibiting prolactin.
What is Hope Medicine Incorporated?
Hope Medicine Inc. is a Chinese biopharmaceutical company founded by Professor Rui Ping Xiao of Peking University. According to their website, Hope Medicine Inc. was founded on the basis of a thorough understanding of disease biology and the practical application of medical research.[3]Hope Medicine, (no date), About Hope Medicine. Available at: https://www.hopemedinc.com/ (Accessed: 21 April 2023) Hope Medicine Inc. focuses on developing small molecule drugs that target specific proteins or pathways involved in disease.
In 2019, Hope Medicine Inc. announced a license agreement with Bayer AG in which Hope Medicine will develop and commercialize HMI-115 as a treatment for male and female pattern hair loss, endometriosis, and other conditions.[4]Cision, (no date), Hope Medicine announces global license agreement with Bayer AG to advance the development and commercialization of the monoclonal antibody directed against prolactin (PRL) … Continue reading
In 2022, Hope Medicine announced that the US Food and Drug Administration (FDA) had approved an application for HMI-115 to be used as an investigational new drug for the treatment of androgenetic alopecia or pattern hair loss, meaning that phase 2 clinical trials can begin in the US. Hope Medicine stated that the company’s plans for its phase 2 trial for androgenetic alopecia would be an international multi-center, randomized, double-blind, placebo-controlled study. Participating countries include the US, Australia, and others. [5]Cision, (no date), Hope Medicine announces a global license agreement with Bayer AG to advance the development and commercialization of the monoclonal antibody directed against prolactin (PRL) … Continue reading
Are these trials likely to work? Let’s get into the science of how this prolactin receptor antagonist, HMI-115, might function. First, we’ll take a deep dive into what prolactin is and how it might affect hair follicle biology.
What is Prolactin?
Prolactin is a hormone produced and secreted by the anterior pituitary gland. Prolactin is named for its stimulatory effect on lactation (milk production), but in actuality, prolactin has many different functions in both men and women.[6]Freeman, M.A., Kanyicska, B., Lerant, A., Nagy, Gyorgy. (2000). Prolactin: structure, function, and regulation of secretion. Physiological Reviews. 80(4), 1523-1631. Available at: … Continue reading The normal range of prolactin in non-pregnant women is below 25 ng/ml and in men, below 20 ng/ml.[7]Majumdar, A., Mangal, N.S. (2013). Hyperprolactinemia. Journal of Human Reproductive Sciences. 6(3). 168-175. Available at: https://doi.org/10.4103/0974-1208.121400
High levels of prolactin can lead to a medical condition called hyperprolactinemia. Symptoms of hyperprolactinemia can include irregular menstrual periods/loss of menstrual periods in women, decreased sex drive and erectile dysfunction in men, infertility, overproduction of breast milk in non-pregnant or non-breastfeeding women, and also osteoporosis.[8]Capozzi, A., Scambia, G., Pontecorvi, A., Lello, S. (2015). Hyperprolactinemia: pathophysiology and therapeutic approach. Gynecological Endocrinology. 31(7). 506-510. Available at: … Continue reading
It is also thought that high prolactin levels may also be involved in hair loss (more on that below).[9]Schmidt, J.B. (1994). Hormonal basis of male and female androgenic alopecia: clinical relevance. Skin Pharmacology. 7. 61-66. Available at: https://doi.org/10.1159/000211275
How is Prolactin Involved in Hair Cycling and Loss?
Prolactin upregulates several molecular signaling pathways that are associated with hair loss. For example, the Janus kinase-signal transducer and activator of transcription (JAK/STAT) pathway is activated by prolactin.[10]Ma, F.Y., Anderson, G.M., Gunn, T.D., Goffin, V., Grattan, D.R., Bunn, S.J. (2005). Prolactin specifically activates signal transducer and activator of transcription 5b in neuroendocrine dopaminergic … Continue reading The JAK/STAT pathway is known to be involved in hair loss in patients with alopecia areata; therefore, elevated prolactin levels may subsequently enhance the JAK/STAT pathway, leading to worse severity of the disease.
Prolactin and prolactin receptors are expressed at both the mRNA and protein levels in the hair follicle. Furthermore, both of these appear to be up-regulated in the late growing stage (anagen) into the transitional stage (catagen) of the hair follicle cycle.[11]Foitzik, K., Krause, K., Conrad, F., Nakamura, M., Funk, W., Paus, R. (2006). Human scalp hair follicles are both a target and a source of prolactin, which serves as an autocrine and/or paracrine … Continue reading The researchers also found that treatment of isolated human hair follicles with high doses of prolactin (around 400 ng/ml) resulted in significant inhibition of hair growth and premature transition into catagen compared to control hair follicles. Additionally, markers for cell growth and cell death were reduced and increased, respectively, following prolactin treatment. Hair follicles were also staged for the hair cycle following prolactin treatment, and there was a significant increase in the number of treated follicles in the catagen stage. These findings indicate that prolactin can impact hair follicle biology and that prolactin may function as an inducer of catagen.

Figure 1: Effect of 400 ng/ml of prolactin on (LEFT): hair follicle growth and (RIGHT): stage of the hair follicle cycle.[12]Foitzik, K., Krause, K., Conrad, F., Nakamura, M., Funk, W., Paus, R. (2006). Human scalp hair follicles are both a target and a source of prolactin, which serves as an autocrine and/or paracrine … Continue reading
In another study, in mice, it was found that prolactin is closely involved in regulating the hair follicle cycle. Prolactin levels increased in mice at 3 weeks, just before the onset of anagen (Figure 2), with a subsequent increase in the prolactin receptor at the start of the anagen stage. This increase in prolactin levels was associated with a concomitant increase in levels of prolactin receptors in the hair follicle. Suppression of prolactin secretion (through oral treatment of a known inhibitor of prolactin called bromocriptine) from the pituitary gland induced hair growth 3-5 days earlier, which was returned to normal by treating the mice with prolactin for 3 days. Increasing the duration of treatment had an inhibitory effect on hair growth.[13]Craven, A.J., Nixon, A.J., Ashby, M.G., Ormandy, C.J., Blazek, K., Wilkins, R.J., Pearson, A.J. (2006). Prolactin delays hair regrowth in mice. Journal of Endocrinology. 191. 415-425. Available at: … Continue reading

Figure 2: Levels of circulating prolactin in mice. Prolactin levels appear to increase at around 21 days (3 weeks) of age – around one week before the mice enter the anagen stage of the hair follicle cycle.[14]Craven, A.J., Nixon, A.J., Ashby, M.G., Ormandy, C.J., Blazek, K., Wilkins, R.J., Pearson, A.J. (2006). Prolactin delays hair regrowth in mice. Journal of Endocrinology. 191. 415-425. Available at: … Continue reading
There is some evidence demonstrating that prolactin is involved in regulating hair follicle cycling. However, is prolactin relevant to hair loss disorders in humans?
In women, hyperprolactinemia was found to be associated with androgenetic alopecia-pattern hair loss, as well as hirsutism (excess facial hair).[15]Schmidt, J.B. (1994). Hormonal basis of male and female androgenic alopecia: clinical relevance. Skin Pharmacology. 7. 61-66. Available at: https://doi.org/10.1159/000211275 A study involving 65 males (mean age of 24) and 46 females (mean age of 34) with androgenetic alopecia was conducted, and levels of different hormones were measured in comparison to 58 male and 45 female healthy controls. Prolactin, however, was only measured in the females in the study. Using a TRH test – which stimulates prolactin release from the pituitary – the researchers measured the levels of TRH-induced prolactin in both hair loss patients and controls (Figure 3). The researchers wanted to see whether the total stores of prolactin were increased in those with hair loss or whether the prolactin system was hypersensitive in some way. The female Patients were administered 0.2 mg of TRH and then had blood taken after 0 minutes, 20 minutes, and 40 minutes of treatment. Prolactin levels were then measured. This was taken during the luteal phase of the menstrual cycle (around day 14 to 28) when prolactin levels were higher than normal. It is important to note, however, that prolactin levels can be highly dynamic.
The researchers found an increase in prolactin levels at minute 0 and minute 20 following TRH administration in patients with androgenetic alopecia compared to the control patients, but these results were not statistically significant. However, there was a significant increase in prolactin levels after 40 minutes of TRH administration (p=<0.05). In conclusion, the findings of this study suggest that prolactin levels may be elevated in patients with androgenetic alopecia.

Figure 3: Median basal and stimulated levels of prolactin (PRL) and thyroid stimulating hormone (TSH), 0 minutes, 20 minutes, and 40 minutes after stimulation with TRH in female hair loss patients and controls. Adapted from:[16]Schmidt, J.B. (1994). Hormonal basis of male and female androgenic alopecia: clinical relevance. Skin Pharmacology. 7. 61-66. Available at: https://doi.org/10.1159/000211275
Another study looked at 40 women (aged between 30 and 65) with moderately increased prolactin levels, alongside androgenetic alopecia or diffuse hair loss, to determine the effects that prolactin might have on hair loss.[17]Lutz, G. (2011). Hair loss and hyperprolactinemia in women. Dermato-Endocrinology. 4(1). 70-76. Available at: https://doi.org/10.4161/der.19472 Prolactin levels varied widely in these patients, with the lowest level being 25.1 ng/ml and the highest being 1390 ng/ml. Interestingly, there did not appear to be any correlation between the number of non-growing hairs and prolactin levels, leading the researchers to conclude that it was not likely that elevated prolactin levels were the cause of diffuse or androgenetic alopecia (Figures 4 & 5).

Figure 4: Prolactin levels in patients with either diffuse or androgenetic alopecia. Adapted from:[18]Lutz, G. (2011). Hair loss and hyperprolactinemia in women. Dermato-Endocrinology. 4(1). 70-76. Available at: https://doi.org/10.4161/der.19472

Figure 5: Number of telogen hairs of patients with either diffuse or androgenetic alopecia. Vertical axis = the number of telogen hairs; Horizontal axis = patient. Adapted from: [19]Lutz, G. (2011). Hair loss and hyperprolactinemia in women. Dermato-Endocrinology. 4(1). 70-76. Available at: https://doi.org/10.4161/der.19472
A case-control study of 30 patients (16 men and 14 women) aged between 18-46 with alopecia areata (AA) and 20 controls (9 males and 11 females) aged between 18-45 was undertaken.[20]Tahlawi, S.M.E., Eishi, N.H.E., Kahhal, R.K., Hegazy, R.A., Hanafy, G.M.E., Hay, R.M.A., Shaker, O.G. (2018). Do prolactin and its receptor play a role in alopecia areata? Indian Journal of … Continue reading When comparing the serum prolactin level between AA patients and controls using an ELISA assay, no significant differences were found. PCRs were then completed with RNA harvested from biopsies taken from the lesional skin of AA patients and the occipital scalp skin of controls. An increase in the level of prolactin receptors was observed in the AA patients compared to the control patients (Figure 6). This indicates a potential hypersensitivity to prolactin in patients with AA rather than excess prolactin.

Figure 6: Comparison of the mean serum prolactin level and mean tissue prolactin receptor levels in AA patients vs. control patients. Adapted from:[21]Tahlawi, S.M.E., Eishi, N.H.E., Kahhal, R.K., Hegazy, R.A., Hanafy, G.M.E., Hay, R.M.A., Shaker, O.G. (2018). Do prolactin and its receptor play a role in alopecia areata? Indian Journal of … Continue reading
Furthermore, the levels of prolactin receptors correlate with the SALT score (Severity of Alopecia Tool) – a common score used in studies to assess the extent of scalp hair loss.
Overall, some evidence implicates prolactin in the pathogenesis of androgenetic alopecia. However, there are some contradicting findings about whether prolactin is involved in the pathogenesis of hair loss. Moreover, one might expect there to be more of a concrete link between pregnancy and hair loss, given the large changes in prolactin that occur during late pregnancy and throughout breastfeeding. However, there is little evidence of changes in hair loss and ratios of anagen/telogen hairs between different stages of pregnancy or breastfeeding status.[22]Mirallas, O., Grimalt, R. (2016). The postpartum telogen effluvium fallacy. 1(4), 198-201. Available at: https://doi.org/10.1159/000445385
However, given the caveats above, let us assume for a moment that prolactin is a definitive cause of hair loss, so why not just use already-known treatments for hyperprolactinemia, such as the drug cabergoline?
A major mechanism regulating prolactin levels is dopamine secretion by neurons in the hypothalamus. Dopamine then inhibits the secretion of prolactin from the pituitary. Cabergoline is a dopamine agonist and so promotes the action of dopamine. Some case studies have indicated that dopamine agonists like cabergoline may cause hair loss in some people. One such study included a 71-year-old woman with Parkinson’s disease who experienced progressive hair loss after taking 3 mg of cabergoline for one month. After 4 months of decreasing the dose, however, (from 3 mg to 1 mg), the patient’s hair was completely re-grown.[23]Miwa, H., Kondo, T. (2003). Hair loss induced by dopamine agonist: case report and review of the literature. Parkinsonism & Related Disorders. 10. 51-52. Available at: … Continue reading An earlier study also reports two women suffering from diffuse alopecia as a result of taking levodopa (dopamine replacement) as a treatment for Parkinson’s.[24]Marshall, A., Williams M.J. (1971). Alopecia and levodopa. British Medical Journal. 2(5752), 47. Available at: https://doi.org/10.1136/bmj.2.5752.47 Whether dopaminergic drugs could benefit hair loss in otherwise healthy people is unclear. In any case, given the widespread importance of dopamine in regulating mood and behavior, targeting this system as a therapy for hair loss may not be a wise strategy. Therefore, other pharmaceutical approaches, like the blockade of prolactin receptors, may be a better method. That is if prolactin is indeed related to hair loss.
So, let’s have a look at whether there is any evidence currently to suggest that the prolactin receptor antagonist HMI-115 might benefit those with hair loss.
Could HMI-115 work for Patients with Androgenetic Alopecia?
Recent data showing the potential effects of HMI-115 in humans has been released, but let’s first look at the published animal data.
Animals
Bayer AG did complete a study using a primate model of androgenetic alopecia, which Bayer AG goes into detail about in their patent application.[25]Ekkehard, M., Joachim, S., Jorn, K., Christiane, O. (2017). Prolactin receptor antibody for male and female pattern hair loss. World Intellectual Property Organization. Available at: … Continue reading Unfortunately, this study does not appear to be published anywhere other than in the patent application that we found on Google (such as in a peer-reviewed scientific journal).
This study attempted to leverage an apparent phenomenon of spontaneous post-adolescence scalp hair loss in stump-tailed macaques as a model system to test the effects of HIM-115. Over a period of 6 months, the macaques were treated with HMI-115. The researchers claim that terminal hairs nearly doubled throughout the treatment. Unfortunately, the data is only given in terms of change from baseline, and there does not seem to be an untreated control group, which makes it difficult to conclude whether HMI-115 is doing anything (Figure 7).

Figure 7: Effect of HMI-115 treatment on Hair Growth in Stump-Tailed Macaques with Androgenetic Alopecia over 24 weeks. Adapted from:[26]Ekkehard, M., Joachim, S., Jorn, K., Christiane, O. (2017). Prolactin receptor antibody for male and female pattern hair loss. World Intellectual Property Organization. Available at: … Continue reading
Humans
There are two clinical trials currently recruiting for HMI-115: one phase 2 trial for endometriosis-associated pain and another phase 2 trial for treating male androgenetic alopecia over 24 weeks.[27]Clinical Trials, (no date). HMI-115. Available at: https://clinicaltrials.gov/ct2/results?cond=&term=HMI-115&cntry=&state=&city=&dist= (Accessed: 02 May 2024) The latter of the two follows on from a now complete phase 1 trial, whereby HMI-115 was tested as a treatment for male and female androgenetic alopecia.[28]Hope Medicine. (2023). An Open Label Study, to Evaluate Safety, Tolerability, and Efficacy in Male and Female With Androgenetic Alopecia Treated With HMI-115 Over a 24-Week Treatment Period (Clinical … Continue reading Importantly, this means that we have some information regarding the efficacy and safety of HMI-115 in humans – here’s what they found.
The study investigated the potential of HMI-115 as a treatment for both male and female androgenetic alopecia. 12 male and 4 female participants were given 240mg of HMI-115 every two weeks by subcutaneous injection for 24 weeks. The primary measurement was a change in target area hair count (TAHC) of non-vellus hair from baseline (week 0) to week 24.
In a press release, Hope Medicine Inc. (the sponsor of the trial) stated that “HMI-115 demonstrated positive efficacy results. It is also safe and well-tolerated”.[29]Hope Medicine. (No date). Positive Outcome from a Phase Ib Study in Australia Treating Patients with Androgenic Alopecia. Hope Medicine Available at: https://www.hopemedinc.com/company-release-37 … Continue reading. The press release also showed that the mean non-vellus TAHC increased by 14 hairs/cm2 in the male group, which was reported as a statistically significant increase. Hope Medicine Inc. acknowledges that this is the first indication that a prolactin blockade receptor may promote hair growth in patients with androgenetic alopecia, potentially opening the door for its use as a novel therapeutic.
While this does sound promising, there are several drawbacks. First and foremost, these results have (at the time of writing) only been shared as a press release. This means that the study and the results have not been peer-reviewed. In other words, no external reviewers have evaluated the study and ensured its integrity.[30]Kelly, J., Sadeghieh, T., Khosrow, A. (2014). Peer Review in Scientific Publications: Benefits, Critiques, & A Survival Guide. eJIFCC. 25(3). 227-243. PMID: 27683470 The lack of peer review means that we can’t be sure that unwanted results have not been omitted. Of the single measurement that has been reported, change in non-vellus TAHC, it is difficult to contextualize an increase of 14 hairs/cm2 – is that a large, moderate, or small change?
Furthermore, the study was conducted on a small number of participants. Generally speaking, the greater the sample size, the more reliable the results. In testing just 12 males and 4 females, the statistical power is low, and there is a risk of obtaining false-positive and false-negative results.[31]Andrade, C., (2020). Sample Size and its Importance in Research. Indian Journal of Psychological Medicine. 42(1). 102-103. Available at: https://doi.org/10.4103/IJPSYM.IJPSYM_504_19
On the one hand, it can be difficult to determine outliers, subjects who differ greatly from the average. For example, a few hyper-responders could see huge increases in their TAHC and cause the mean change of the group to be significant, while the majority of the subjects see no change. On the other hand, small sample sizes can obscure meaningful changes and smaller positive effects that do not reach significance. In either case, small sample sizes do not offer a good estimation of how the treatment may perform in the wider population. Furthermore, Hope Medicine didn’t include any results for the 4 women included, but it is unlikely that there would have been any statistically significant differences anyway.
With that said, HMI-115 does seem to have produced some positive effects, and the phase 2 trial should address some of the issues that hold back its phase 1 counterpart.
What are the Chances of HMI-115 Making it Through Phase 2 Clinical Trials?
So, as mentioned, Hope Medicine Inc. has been approved by the FDA to conduct Phase 2 clinical trials, and they are currently attempting to recruit 180 males with androgenetic alopecia for another 24-week study.
This may sound promising, but, in actual fact, phase 2 trials are generally considered to be the biggest milestone in drug development. The biggest reasons that phase 2 testing might fail are:
- Previously unknown serious side effects occur
- The trial shows insufficient efficacy – i.e., it doesn’t work as well as the researchers initially thought
- Poor commercial viability
It should also be noted that more than 30% of drugs entering phase 2 studies fail to progress, and more than 58% of drugs that do progress then go on to fail in phase 3 trials.[32]Norman, G.A.V. (2019). Phase II trials in drug development and adaptive trial design. JACC Basic to Translational Science. 4(3). 428-437. Available at: https://doi.org/10.1016/j.jacbts.2019.02.005
While the odds are stacked against anyone trying to get their drug through phase 2 clinical trials, the phase 1 trial did provide some hope for HMI-115. Although the study has a number of drawbacks, if the positive effect seen in the males is true (and that’s a big ‘if’), then it provides the best (and only) indication thus far that prolactin receptor blockades may be compatible as a treatment for androgenetic alopecia.
Is HMI-115 Safe?
A safety study was conducted by Bayer AG in a randomized controlled trial with 29 healthy postmenopausal women.[33]Nave, R., Jodi, S., Hoffman, A., Gashaw, I., Zollmann, F., Berse, M., Hochel, J., Kratzschmar, J., Rohde, B. (2019). Monoclonal antibody against prolactin receptor: a randomized placebo-controlled … Continue reading
Participants were split into four groups and administered either HMI-115 or a placebo via subcutaneous injection. Treatments were administered either once every two weeks or once every 3 weeks. The treatment doses were 30 mg (14-day interval), 60 mg (28-day interval), or 90 mg (14-day interval) of HMI-115 or a placebo control on three occasions.
The most frequently occurring adverse events in more than 10% of all study participants (including placebo) were headache, nasopharyngitis (common cold), nausea, lower abdominal pain, injection site bruises, general weakness, back pain, hot flush, oral herpes, temperature increase, and pain in extremities. The adverse events in more than 10% of participants that were associated with HMI-115 were headache, lower abdominal pain, hot flush, and nausea. Compared to the placebo, the frequency of adverse events was not increased with HMI-115, and no dose-dependent increase in adverse event frequency was observed.
Furthermore, the treatment was slowly absorbed, reaching peak concentrations approximately 5 – 11 days after dosing. After the final 90 mg dose, HMI-115 was eliminated from the blood plasma around 16 days later. The researchers determined that the three different dosages of HMI-115 were well-tolerated in healthy postmenopausal women, with no allergic reactions seen.
It remains to be seen if HMI-115 is safe in the long term, or across a larger, more representative group of patients. Furthermore, this study was completed in the context of treating endometriosis; therefore, the number of treatments, the concentration of the drug, and the duration of treatment time may differ from those who may be administered this drug for hair loss disorders.
For comparison, the completed phase 1 clinical trial, which Hope Medicine Inc. sponsored, treated patients with androgenetic alopecia 240 mg of HMI-115 every 2 weeks via subcutaneous injection, which is much higher than the concentrations used in the safety study completed by Bayer.[34]Clinical Trials, (no date). HMI-115. Available at: https://clinicaltrials.gov/ct2/results?cond=&term=HMI-115&cntry=&state=&city=&dist= (Accessed: 21 April 2023) Hope Medicine Inc. reported that this delivery of HMI-115 proved safe and tolerable, though they did not provide any data to support this statement.
Is HMI-115 for Me?
Well, HMI-115 is not yet available, and it looks like it won’t be available for another few years, at least as we look to the beginning of the phase 2 trials. While it’s still too early to tell if this product will make it to the market, we look forward to seeing the full results from the phase 1 trial and those from further trials to come.
References[+]
References ↑1 Hope Medicine, (no date). Clinical Development. Research and Development. Available at: https://www.hopemedinc.com/research-platform?tp2 (Accessed: 21 April 2023) ↑2 Ferraris, J., Bernichten, S., Pisera, D., Goffin, V. (2013). Use of prolactin receptor antagonists to better understand prolactin regulation of pituitary homeostasis. Neuroendocrinology. 98. 171-179. Available at: https://doi.org/10.1159/000354701 ↑3 Hope Medicine, (no date), About Hope Medicine. Available at: https://www.hopemedinc.com/ (Accessed: 21 April 2023) ↑4 Cision, (no date), Hope Medicine announces global license agreement with Bayer AG to advance the development and commercialization of the monoclonal antibody directed against prolactin (PRL) receptor. CISION PR Newswire. Available at: https://en.prnasia.com/releases/apac/hope-medicine-announced-global-license-agreement-with-bayer-ag-to-advance-the-development-and-commercialization-of-the-monoclonal-antibody-directed-against-prolactin-prl-receptor-242094.shtml (Accessed: 21 April 2023) ↑5 Cision, (no date), Hope Medicine announces a global license agreement with Bayer AG to advance the development and commercialization of the monoclonal antibody directed against prolactin (PRL) receptor. CISION PR Newswire. Available at: https://en.prnasia.com/releases/apac/hope-medicine-announced-global-license-agreement-with-bayer-ag-to-advance-the-development-and-commercialization-of-the-monoclonal-antibody-directed-against-prolactin-prl-receptor-242094.shtml (Accessed: 21 April 2023) ↑6 Freeman, M.A., Kanyicska, B., Lerant, A., Nagy, Gyorgy. (2000). Prolactin: structure, function, and regulation of secretion. Physiological Reviews. 80(4), 1523-1631. Available at: https://doi.org/10.1152/physrev.2000.80.4.1523 ↑7 Majumdar, A., Mangal, N.S. (2013). Hyperprolactinemia. Journal of Human Reproductive Sciences. 6(3). 168-175. Available at: https://doi.org/10.4103/0974-1208.121400 ↑8 Capozzi, A., Scambia, G., Pontecorvi, A., Lello, S. (2015). Hyperprolactinemia: pathophysiology and therapeutic approach. Gynecological Endocrinology. 31(7). 506-510. Available at: https://doi.org/10.3109/09513590.2015.1017810 ↑9, ↑15, ↑16 Schmidt, J.B. (1994). Hormonal basis of male and female androgenic alopecia: clinical relevance. Skin Pharmacology. 7. 61-66. Available at: https://doi.org/10.1159/000211275 ↑10 Ma, F.Y., Anderson, G.M., Gunn, T.D., Goffin, V., Grattan, D.R., Bunn, S.J. (2005). Prolactin specifically activates signal transducer and activator of transcription 5b in neuroendocrine dopaminergic neurons. Endocrinology. 146(12). 5112-5119. Available at: https://doi.org/10.1210/en.2005-0770 ↑11, ↑12 Foitzik, K., Krause, K., Conrad, F., Nakamura, M., Funk, W., Paus, R. (2006). Human scalp hair follicles are both a target and a source of prolactin, which serves as an autocrine and/or paracrine promoter of apoptosis-driven hair follicle regression. The American Journal of Pathology. 168(3). 748-756. Available at: https://doi.org/10.2353/ajpath.2006.050468 ↑13, ↑14 Craven, A.J., Nixon, A.J., Ashby, M.G., Ormandy, C.J., Blazek, K., Wilkins, R.J., Pearson, A.J. (2006). Prolactin delays hair regrowth in mice. Journal of Endocrinology. 191. 415-425. Available at: https://doi.org/10.1677/joe.1.06685 ↑17, ↑18, ↑19 Lutz, G. (2011). Hair loss and hyperprolactinemia in women. Dermato-Endocrinology. 4(1). 70-76. Available at: https://doi.org/10.4161/der.19472 ↑20 Tahlawi, S.M.E., Eishi, N.H.E., Kahhal, R.K., Hegazy, R.A., Hanafy, G.M.E., Hay, R.M.A., Shaker, O.G. (2018). Do prolactin and its receptor play a role in alopecia areata? Indian Journal of Dermatology. 63(3), 241-245. https://doi.org/10.4103/ijd.IJD_590_17 ↑21 Tahlawi, S.M.E., Eishi, N.H.E., Kahhal, R.K., Hegazy, R.A., Hanafy, G.M.E., Hay, R.M.A., Shaker, O.G. (2018). Do prolactin and its receptor play a role in alopecia areata? Indian Journal of Dermatology. 63(3), 241-245. https://doi.org/10.4103/ijd.IJD_590_17 ↑22 Mirallas, O., Grimalt, R. (2016). The postpartum telogen effluvium fallacy. 1(4), 198-201. Available at: https://doi.org/10.1159/000445385 ↑23 Miwa, H., Kondo, T. (2003). Hair loss induced by dopamine agonist: case report and review of the literature. Parkinsonism & Related Disorders. 10. 51-52. Available at: https://doi.org/10.1016/S1353-8020(03)00058-0 ↑24 Marshall, A., Williams M.J. (1971). Alopecia and levodopa. British Medical Journal. 2(5752), 47. Available at: https://doi.org/10.1136/bmj.2.5752.47 ↑25 Ekkehard, M., Joachim, S., Jorn, K., Christiane, O. (2017). Prolactin receptor antibody for male and female pattern hair loss. World Intellectual Property Organization. Available at: https://patentimages.storage.googleapis.com/56/cc/a3/7d3ac32f1537b2/WO2019011719A1.pdf (Accessed: 23 April 2023) ↑26 Ekkehard, M., Joachim, S., Jorn, K., Christiane, O. (2017). Prolactin receptor antibody for male and female pattern hair loss. World Intellectual Property Organization. Available at: https://patentimages.storage.googleapis.com/56/cc/a3/7d3ac32f1537b2/WO2019011719A1.pdf (Accessed: 23 April 2023) ↑27 Clinical Trials, (no date). HMI-115. Available at: https://clinicaltrials.gov/ct2/results?cond=&term=HMI-115&cntry=&state=&city=&dist= (Accessed: 02 May 2024) ↑28 Hope Medicine. (2023). An Open Label Study, to Evaluate Safety, Tolerability, and Efficacy in Male and Female With Androgenetic Alopecia Treated With HMI-115 Over a 24-Week Treatment Period (Clinical Trial Registration NCT05324293). Clinical Trials. (Accessed: 02 May 2024) ↑29 Hope Medicine. (No date). Positive Outcome from a Phase Ib Study in Australia Treating Patients with Androgenic Alopecia. Hope Medicine Available at: https://www.hopemedinc.com/company-release-37 (Accessed 2 May 2024) ↑30 Kelly, J., Sadeghieh, T., Khosrow, A. (2014). Peer Review in Scientific Publications: Benefits, Critiques, & A Survival Guide. eJIFCC. 25(3). 227-243. PMID: 27683470 ↑31 Andrade, C., (2020). Sample Size and its Importance in Research. Indian Journal of Psychological Medicine. 42(1). 102-103. Available at: https://doi.org/10.4103/IJPSYM.IJPSYM_504_19 ↑32 Norman, G.A.V. (2019). Phase II trials in drug development and adaptive trial design. JACC Basic to Translational Science. 4(3). 428-437. Available at: https://doi.org/10.1016/j.jacbts.2019.02.005 ↑33 Nave, R., Jodi, S., Hoffman, A., Gashaw, I., Zollmann, F., Berse, M., Hochel, J., Kratzschmar, J., Rohde, B. (2019). Monoclonal antibody against prolactin receptor: a randomized placebo-controlled study evaluating safety, tolerability, and pharmacokinetics of repeated subcutaneous administrations in postmenopausal women. Reproductive Sciences. 26(4). 523-531. Available at: https://doi.org/10.1177/1933719118776806 ↑34 Clinical Trials, (no date). HMI-115. Available at: https://clinicaltrials.gov/ct2/results?cond=&term=HMI-115&cntry=&state=&city=&dist= (Accessed: 21 April 2023) Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Sarah King, PhD
Dr. Sarah King is a researcher & writer who holds a BSc in Medical Biology, an MSc in Forensic Biology, and a Ph.D. in Molecular and Cellular Biology. While at university, Dr. King’s research focused on cellular aging and senescence through NAD-dependent signaling – along with research into prostaglandins and their role in hair loss. She is a co-author on several upcoming manuscripts with the Perfect Hair Health team.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
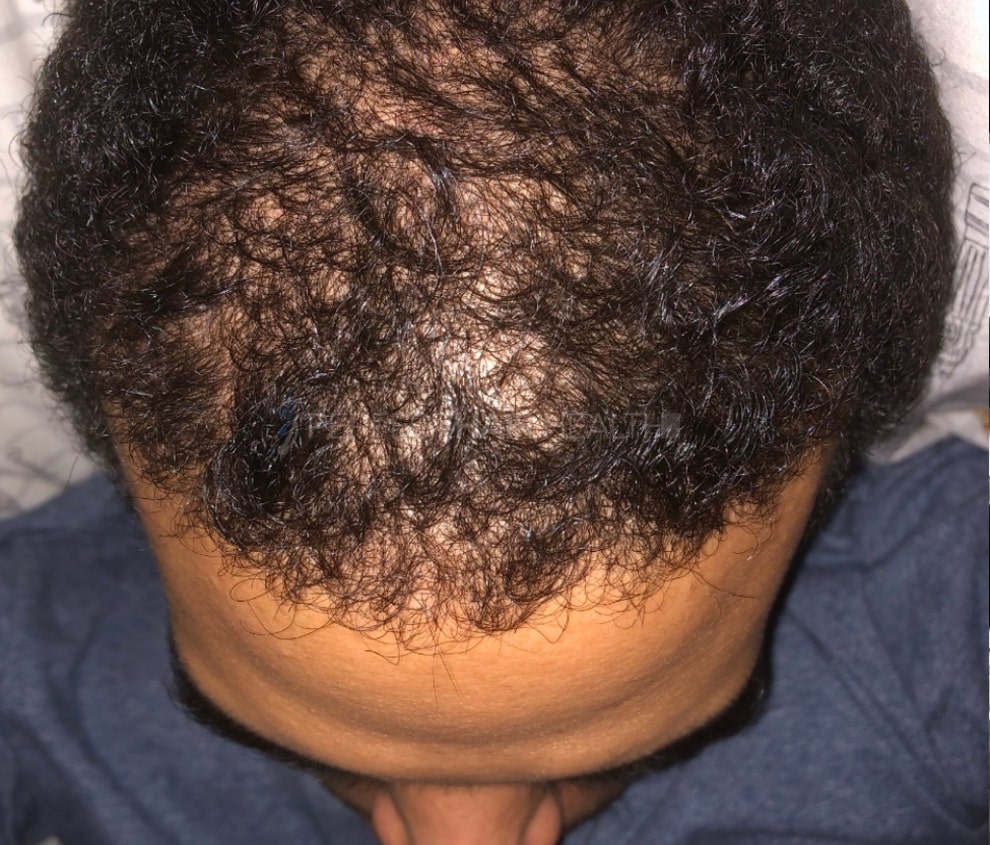
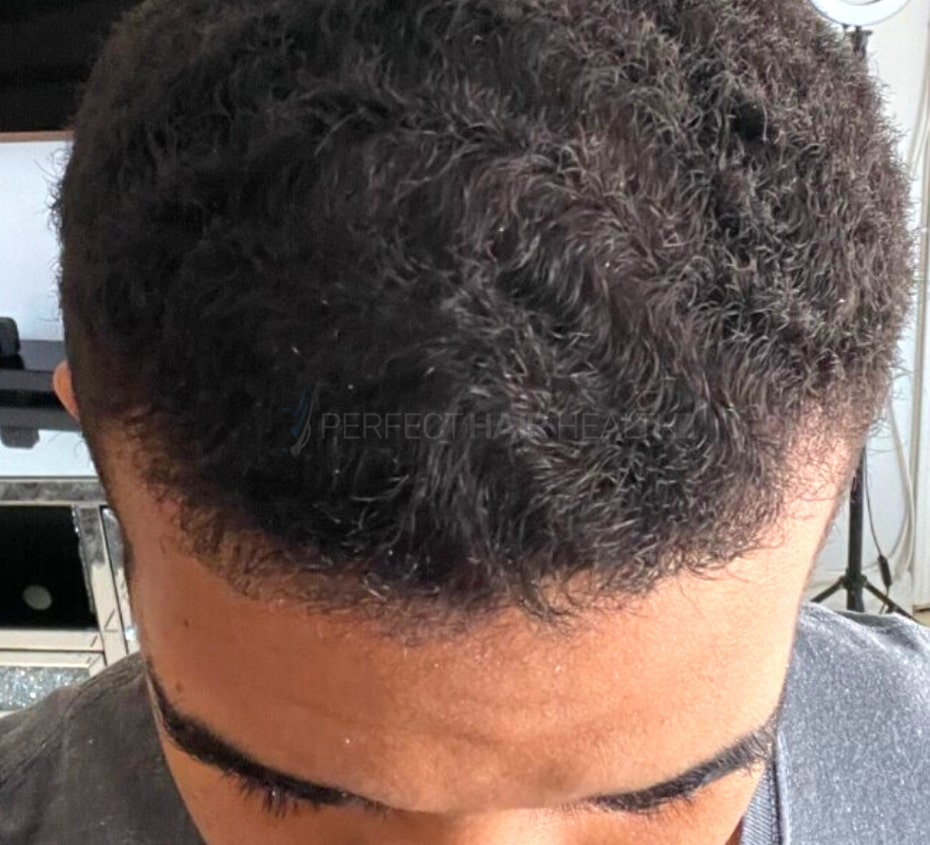 — RDB, 35, New York, U.S.A.
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
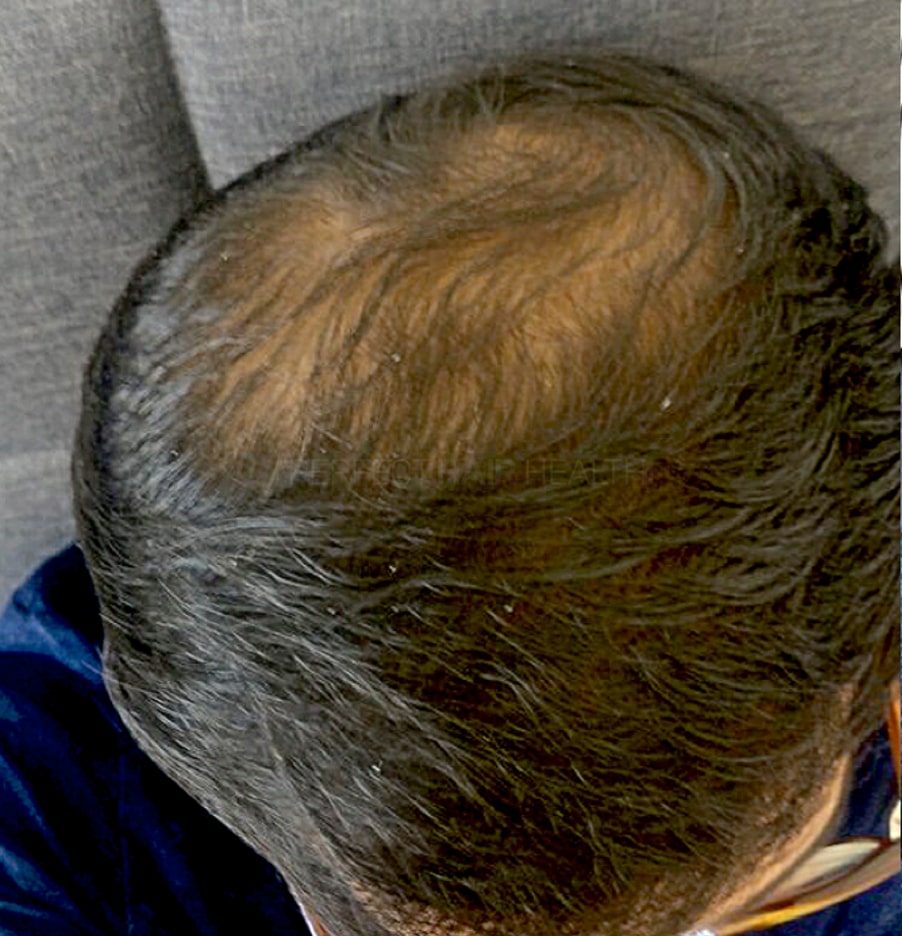
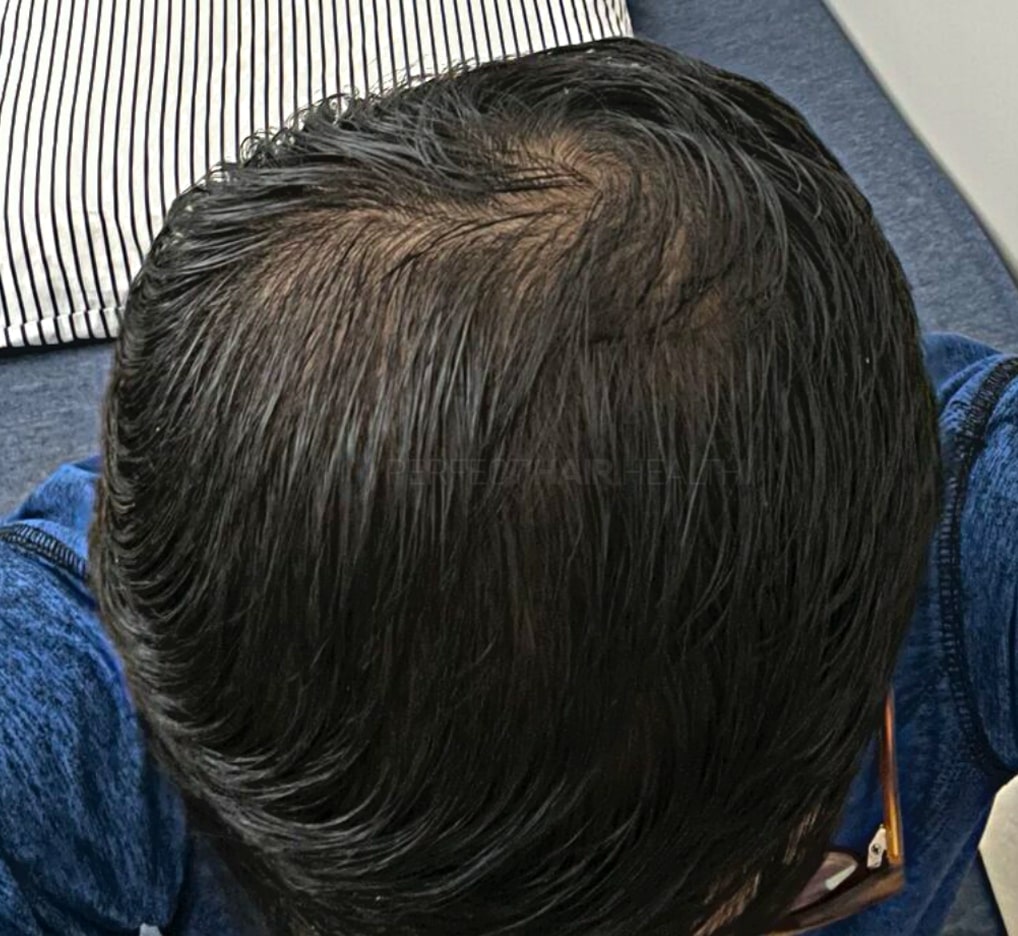 — Aayush, 20’s, Boston, MA
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
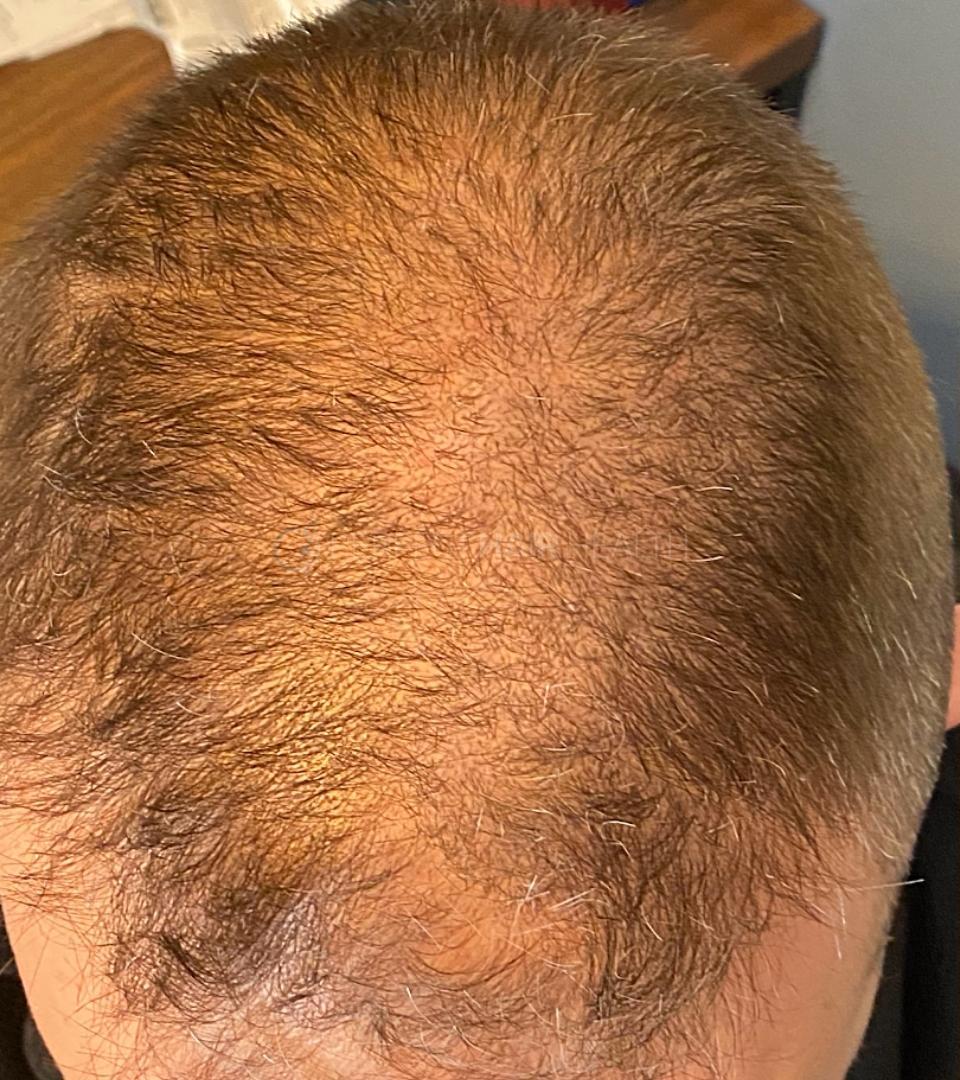
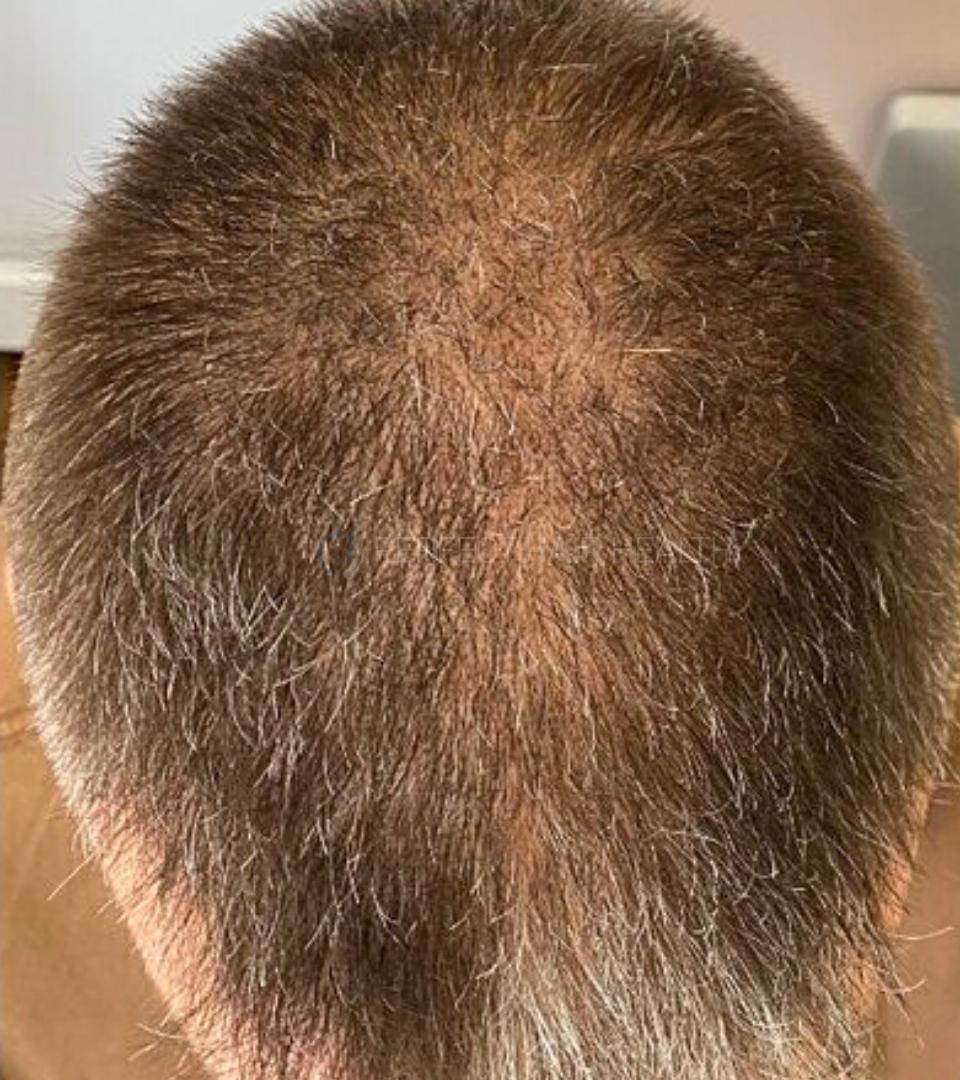 — Douglas, 50’s, Montréal, Canada
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement