- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
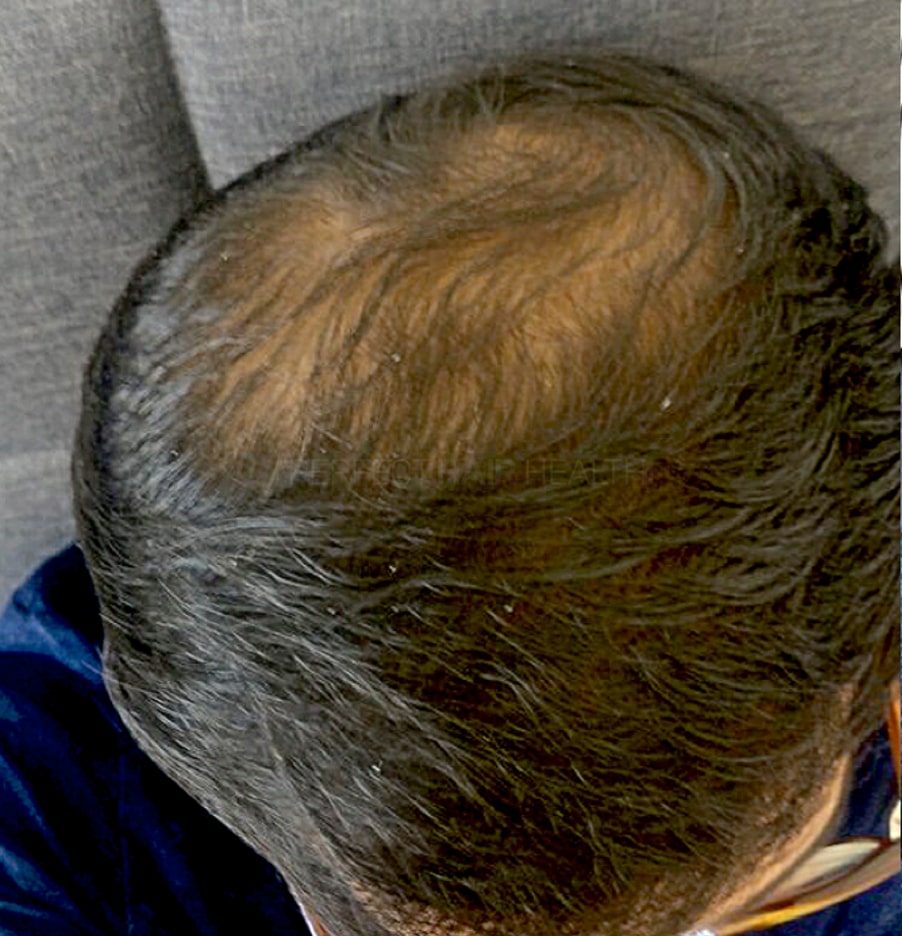
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
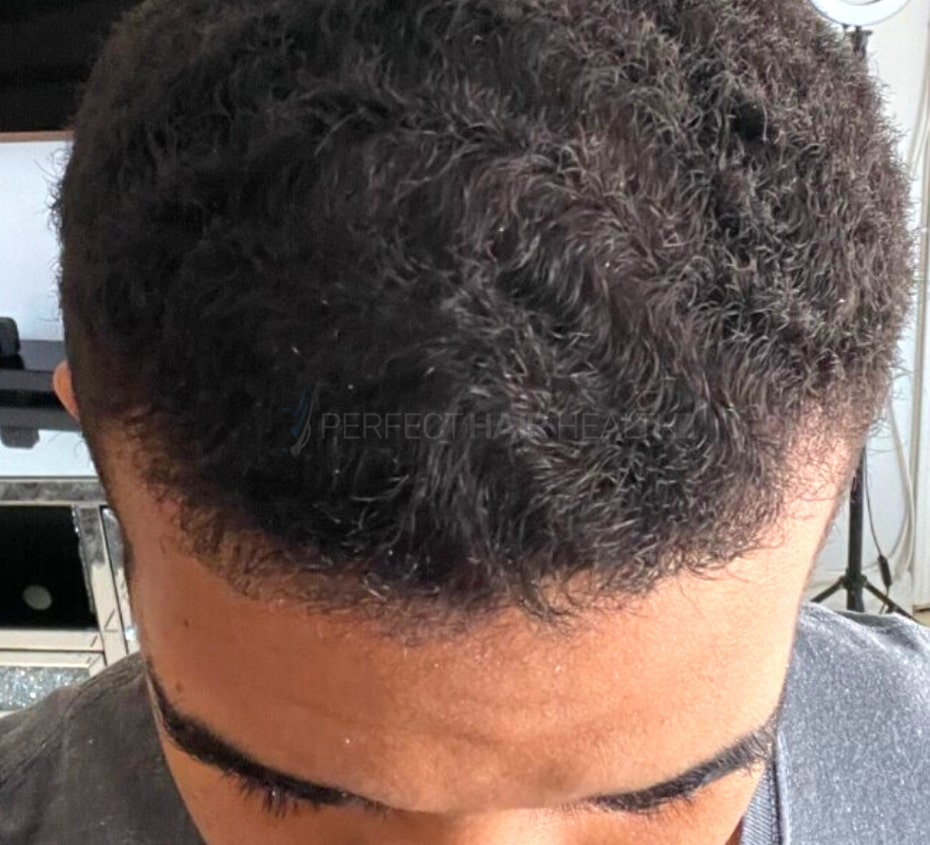
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
 Scroll DownPopular Treatments
Scroll DownPopular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Topical Finasteride Calculator
- Interactive Guide: What Causes Hair Loss?
- Free Guide: Standardized Scalp Massages
- 7-Day Hair Loss Email Course
- Ingredients Database
- Interactive Guide: Hair Loss Disorders
- Treatment Guides
- Product Lab Tests: Purity & Potency
- Evidence Quality Masterclass
More
Articles100+ free articles.
-
Cannabidiol (CBD) Increases Hair Counts By 246%? Not So Fast.
-
Creatine: Does It Worsen Hair Loss? It Depends On The Hair Loss Type.
-
Can Progesterone Improve Hair Regrowth?
-
CRABP2: Can This Gene Predict Regrowth From Retinoids?
-
BTD: Can This Gene Predict Regrowth From Biotin?
-
COL1A1: Can This Gene Predict Regrowth From Collagen Support?
-
2dDR For Hair Loss: What Do We Know So Far About This Sugar?
-
CYP19A1: Can This Gene Predict Regrowth From Hormone Therapy?
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
ArticlesGenes & Hair Loss: Is Balding Inevitable?
First Published Jan 18 2017Last Updated Oct 29 2024IngredientsNatural Remedies Researched & Written By:Perfect Hair Health Team
Researched & Written By:Perfect Hair Health Team Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
Is hair loss caused by genetics? Oftentimes, the answer is yes. But that doesn’t mean that genetic predisposition = genetic destiny. Hair loss treatments have demonstrated an ability to alter the epigenetics of our hair follicles – dampening genes linked to balding and amplifying genes that may protect against androgenic alopecia. This article uncovers some of the science.
Full Article
“You’re losing your hair? Well, it’s genetic.” This is what most people hear after asking a medical professional what causes hair loss.
While there’s absolutely truth to this statement, learning that hair loss is genetic often makes people feel as if going bald is also out of their control. But this couldn’t be further from the truth.
While certain genes may predispose men and women to hair loss, not everyone with these genes will go bald. And even if you’re thinning and have hair loss-related genes, it doesn’t mean that baldness is your genetic destiny.
Genetics & Hair Loss: Fact Or Fiction?
After my hair loss diagnosis, every medical practitioner I ever consulted told me I was losing my hair because of my genes. Even my physician said that while drugs like Rogaine or Propecia may slow or reverse some hair loss, there’s not much I can do (short of a hair transplant) to completely reverse the process. In other words, it’s inevitable. It’s unstoppable. As my former barber explained to me, “You can’t fight genetics.”
So, is the assumption that baldness is caused by your genes true?
Twenty years ago, the answer was yes. At that time, medical professionals agreed that your genes – or the DNA your parents pass on to you – determine all of biology (from how tall you are to how much hair you have). And since genes “run in the family”, if your father had a heart attack, you’re genetically predisposed to heart disease. If your father lost his hair, you’re genetically predisposed to baldness.
For instance, studies show a clear relationship between certain genes and hair loss. If we have this genotype, we’re twice as likely to go bald. If we’re really unlucky and have this genotype, we’re seven times more likely to bald. If we have both genes? We’re screwed. Or at least that was the assumption.
Today, researchers have continued to make progress identifying sets of genes closely associated with the balding process. At the same time, the gene-hair loss argument isn’t nearly as straightforward as scientists had once hoped. In fact, newer research is now helping rewrite much of what we thought we knew about the relationship between genes, disease states, chronic conditions… and even our hair.
It all began with The Human Genome Project.
Genetic Destiny: The Human Genome Project
In 1990, a series of international research teams began working on a multi-year, multi-billion dollar research collaboration: The Human Genome Project. The objective: to identify and quantify every single human gene. The rationale behind its funding: One major scientific assumption, and one major scientific promise.
The assumption: our genes are the root cause of our cancers, autoimmune disorders, and chronic conditions.
The promise: if we map every single human gene, we can identify the genes that trigger diseases like multiple sclerosis or Parkinson’s. If we know which genes cause disease, we can develop technologies to “delete” those genes and then prevent them from ever happening.
It’s no surprise that the US spearheaded this research project. Over 130 million Americans – or 45% of the entire US population – suffer from chronic diseases. Chronic conditions are responsible for seven out of ten American deaths. They account for over 80% of all US hospital admissions. And every 30 seconds, an American-based doctor amputates a limb as a consequence to one of the country’s most common ailments – diabetes.
Why wouldn’t a disease-ridden nation support a project with such potential? Why wouldn’t this nation want to be at the forefront of these discoveries?
The Human Genome Project’s expectations could not have been higher. Whereas mice have about 20,000 genes, scientists speculated that humans, due to their complexity, would have seven times that amount – 140,000 genes. Once all the genetic triggers of cancers, autoimmunity, and even autism were mapped, we’d begin creating technology to “turn off” those genes and paint a disease-free future.
Thirteen years later, the project was completed. What were the results?
The Human Genome Project’s Promises Fall Flat
The discoveries stemming from the Human Genome Project were puzzling at best, and disappointing at worst.
For one, scientists uncovered that humans don’t have nearly as many genes as we thought. In fact, humans have just about 20,500 genes. Shockingly, we have only 300 unique genes that distinguish us from a mouse.
But the most surprising discovery was that, despite mapping our entire human genome and identifying some genes linked to disease, nearly all gene variants posed a minimal risk for disease development. In other words, the root cause of most disease was not genetic.
Some genes are associated with disease, but most genes do not cause disease.
Intriguingly, researchers also discovered that our genes weren’t so “one dimensional.” Many genes actually serve multiple functions. They don’t have single-purpose relationships with specific processes – like cancer development or metabolism. If we delete a disease-linked gene, we might never develop that disease, but we might also impair our digestion, brain function, or even immunity. For example, the gene mutation that causes sickle cell disorder also protects people from malaria. Sickle cell disorder is more common in malaria-ridden regions, and many researchers believe this mutation actually helps sufferers survive to adulthood and reproduce, even if the disease shortens lifespan overall.
In short, the Human Genome Project failed to fulfill its promise, but it was still undeniably important. It forced researchers to revise one major scientific assumption…
Genetics do not determine all of biology. Our genes are not always our destiny.
In fact, new research speculates that 70-90% of disease risk isn’t genetic. It’s environmental.
My DNA Test For Hair Loss: 23andMe
Early in this article we mentioned a few genes associated with hair loss. There are actually a lot more. I would know. I seem to have most of them.
In 2015, I decided to discover more about my genes. So I went and got my entire human genome sequenced through a service called 23andMe.
This is how it works: 23andMe mails you a vial. You fill that vial with saliva and mail it back to their lab. 23andMe processes your vial, decodes your human DNA, and sends you back the raw data. The company also provides you with “fun” add-on services – like what percent of Neanderthal you are, where your mother and father come from, if you have any relatives in their database, or if you have any life-threatening genetic variants (that one’s not so fun).
I was more interested in finding out what my raw genetic data said about my potential to develop disease. Specifically, I wanted to know, based on my genes, how likely I was to go bald.
So I uploaded my 23andMe data into a company called Promethease. Promethease cross-references your DNA with all the published studies about your genotypes, then sends you an automated 100+ page report. The report tells you everything from how well you methylate B-vitamins to how quickly you detoxify drugs to your genetic predisposition for certain cancers.
So how did my raw DNA compare to all the studies on genotypes and male pattern baldness?
My Genes & Predisposition To Hair Loss
Here’s a readout of how my DNA stacked up versus all the genotypes linked to hair loss.
Let’s start with the good news. I have one genotype that says I have a reduced risk for baldness:

So far so good! (For anyone who’s curious, you can read the study about that genotype here.)
Now for the bad news.
I have ten other genotypes linked to hair loss. Each one of them increases my risk for balding.
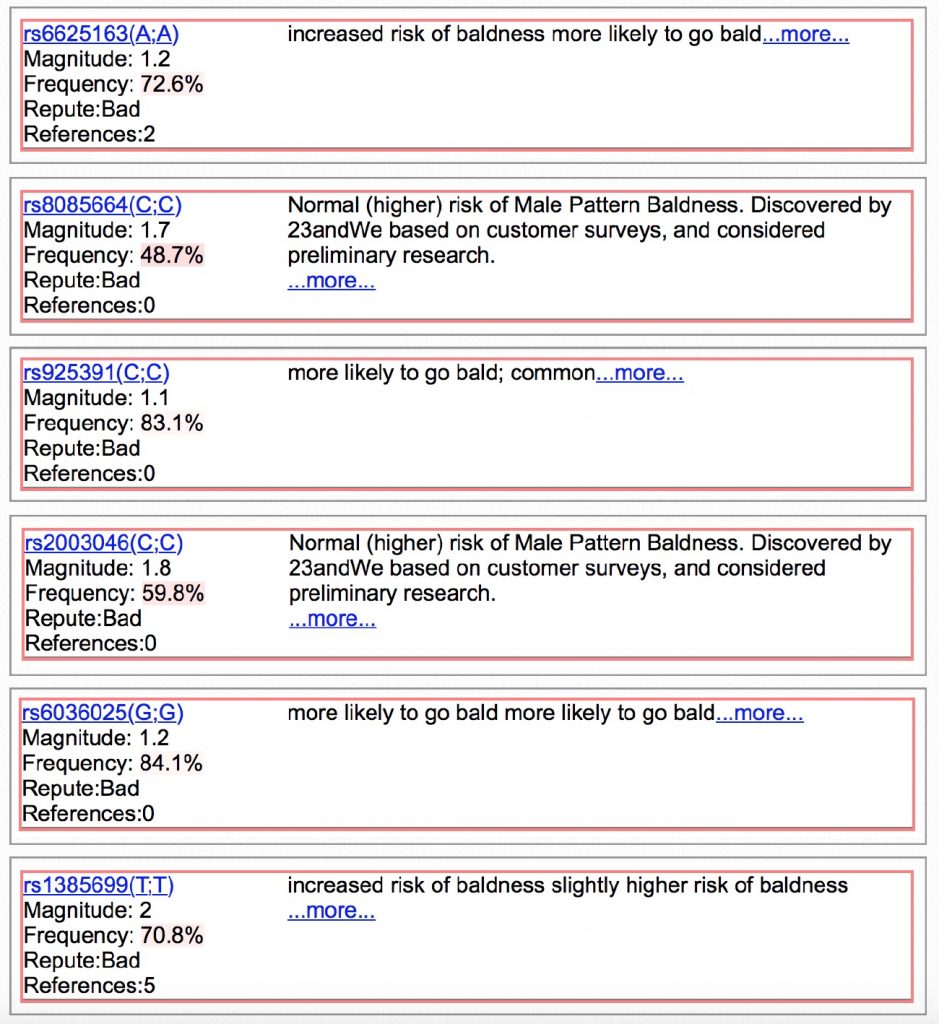
And now for the worst of my genotypes… A 1.6x increased chance for baldness, followed by a 2x increased chance for baldness…
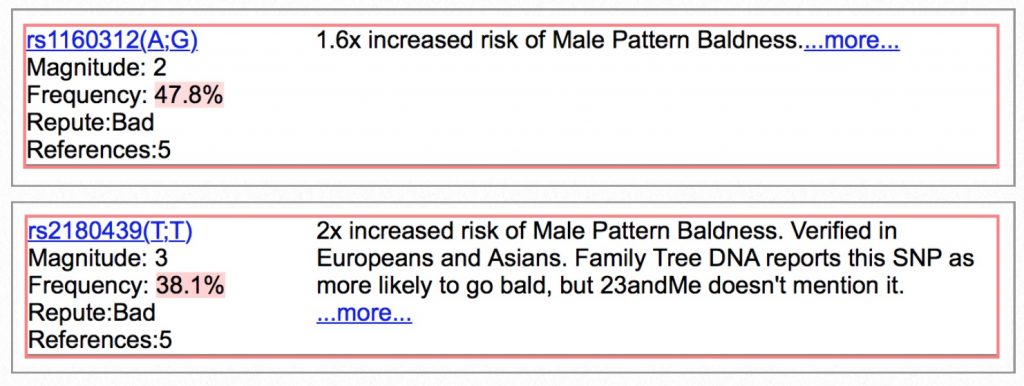
…followed by an increased risk to go completely bald before 40…

…followed by the worst genotype of them all… a 7x greater risk for baldness.
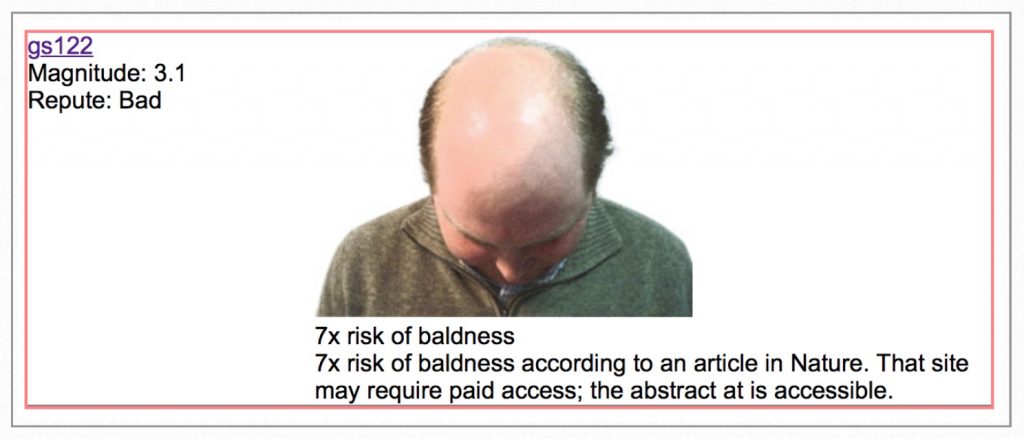
A seven times increased risk for balding! Remember those hair loss genes referenced earlier in the chapter? I have both. Based on my DNA, I’m screwed.
And then there’s the mounting physical evidence.
Most Men In My Family Are Slick Bald
My mother’s father is bald. My mother’s brother is bald. My father’s father was bald. My father’s brother is bald.
And then there’s my hair loss medical diagnosis…
My Hair Loss Medical Diagnosis
I started noticing hair thinning at age 15-16. A few months later, a doctor diagnosed me with male pattern hair loss. Here are his notes from our consultation:


Soon after the diagnosis I picked up a bottle of Rogaine and started an eight-week trial of low-level laser therapy (Luce therapy). While I never followed through with taking Propecia, I ended up taking Rogaine for ~6 years even despite my continued thinning (see my photos).
Now let’s review the facts:
- According to my DNA data, I have a genetic predisposition to pattern hair loss
- At 17 years old, a medical professional diagnosed me with male pattern hair loss
- My 2011 and 2012 photos show clear thinning at the vertex
In other words, my DNA data, diagnosis, family history, and photos all suggest that baldness is my genetic destiny.
And yet today, I’ve still maintained most of my hair. And while I’ve managed to do this without FDA-approved drugs, clinical evidence we have from treatments like minoxidil and finasteride all strongly suggests that long-term maintenance of hair is possible – even when you’re up against genetics.
How is this possible? Well, it turns out that while we can’t change our genes (yet), we do have the power to turn genes “on” or “off”. Researchers now believe disease development has less to do with our genes, and more to do with which genes our body actives or deactivates. This is called gene expression – or when our cells turn combinations of genes on and off to perform certain functions.
Epigenetics has significant relevance to hair loss.
Beyond Genes: Epigenetics
There’s an entire field of study exploring the factors that influence gene expression: Epigenetics. Epigenetics has revolutionized our understanding of disease prevention, pathology, treatment, and reversal.
To understand the potential of epigenetics, let’s look at a few examples of just how easy it is to change gene expression, and just how transformative those changes can be.
We’ll start with agouti mice – a mouse breed once believed to be destined to a life of obesity due to their genes. Then one experiment changed everything.
Epigenetics Breakthrough: The Agouti Mice
Is it possible to stay 100% healthy even if your genes “relegate” you to a life of disease? In the early 2000’s, researchers set out to uncover the answer.
They set up an experiment with a certain type of mice called “agouti” mice. Agouti mice are genetically doomed. They have a mutation in what’s known as the “agouti” gene – a gene that determines fur color. Normal mice vary in color from brown to black. But this mutation makes agouti mice yellow.
But they’re not just yellow. One look at these photo and you’ll notice something else:
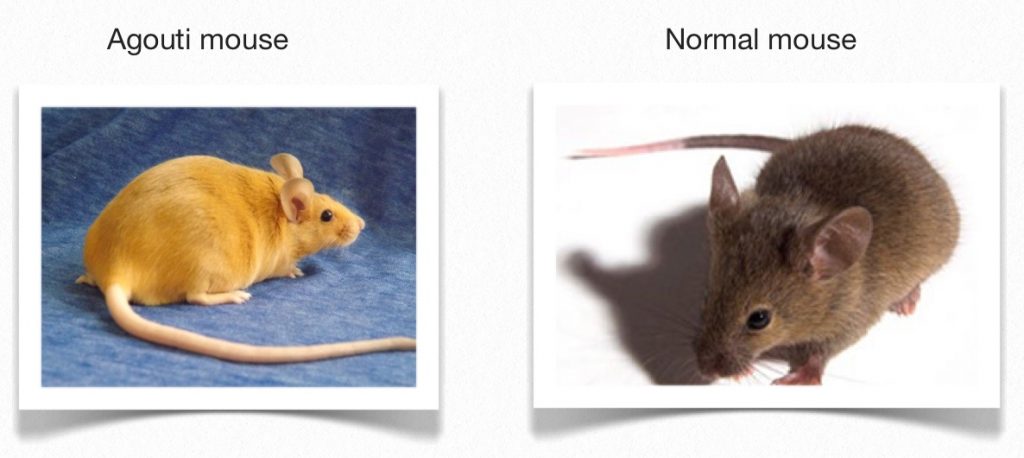
These agouti mice are also obese, ravenous, and far more likely to die prematurely from heart disease, cancer, and diabetes. It’s a mutation that, in extreme cases, is known as a “lethal allele” – a gene variant that causes early death in the animals that have it.
You can’t change your genes. So does this gene guarantee agouti mice to a life of disease?
Researchers decided the best way to answer this was to see if they could influence the agouti gene’s behavior.
The team believed that if they could give mega-doses of certain nutrients to an agouti mouse during its early development (ie: while it was still inside the womb), then maybe those early nutritional gains would help the mouse live longer. Agouti mouse would still be born yellow, but maybe they wouldn’t be so overweight or die so young.
So they split up their agouti female mice into two groups, fed them each two different diets, then impregnated them.
Two Mother Groups, Two Different Diets
One mother group was fed a standard lab rat diet. The other mother group was fed a diet rich in vitamin B-12, folic acid, betaine, and choline for two weeks before and during their pregnancy. The females were mated, and twenty days later, each group was ready to give birth.
This was a longevity study. It would take years for researchers to track each baby’s weight, length, development of cancers, and finally their age of death. In short, no one expected any surprises during the birthing stage. But that’s just what happened.
A Birth Of Surprise
When these agouti mothers gave birth, researchers discovered two things:
- In the group fed a lab rat diet, the babies were born yellow and fat (as expected).
- In the group fed a nutritionally rich diet, the majority of babies were born brown. And skinny.
 (source)
(source)These siblings are genetically identical. How can they look so different?
The Agouti Mice Defy Genetic “Destiny”
The group of agoutis fed mega-doses of nutrients defied genetic destiny. They were supposed to be born yellow and overweight. Instead, they were born brown and skinny – just like mice without the agouti gene mutation.
How is this possible? The agouti gene is unchangeable. It destines agouti mice to be yellow and fat. “You can’t fight genetics,” as my barber said.
Except you can. Here’s something doctors don’t mention about genes: they can become activated or inactivated. That’s what Epigenetics is – the study of gene expression. And this agouti mouse study was a breakthrough. Why? It proved we could control gene expression. We can turn off the gene that makes mice yellow and fat… using only better prenatal nutrition.
This is possible through a process called methylation.
Gene Expression & Methylation: Why Our Bodies Turn Genes On And Off
Before conducting the agouti mice study, researchers uncovered that the agouti gene – the gene responsible for agouti mice’s yellow coat and obesity – was “unmethylated”. What does that mean?
It means in the yellow mice, the agouti gene was always “turned on.” It was always activated inside the mouse’s cells.
Researchers also knew that methyl donors – compounds found in certain foods – could potentially “methylate” certain genes – or turn them off.
How Does Methylation Alter Genes Expression?
To put it simply, inside every cell is DNA, and inside DNA are genes. When we eat food, our cells break down that food for energy. If that food also contains methyl donors, those methyl donors will also enter our cells and leave behind small chemical tags on top of our DNA. These chemical tags tell the cell which genes it should turn on or off.
An unmethylated gene is active – or turned on (upregulated). A methylated gene is turned off – or inactivated (downregulated).
For a fascinating overview, see this flowchart.
These researchers thought that if they fed pregnant agouti mice enough methyl donors, maybe those methyl donors would “methylate” the agouti gene, and in doing so, dim the gene’s effects on the mother’s babies.
They did, and beyond all expectations.
The diets high in methyl donors not only methylated the agouti gene, they turned it off completely. What’s even more interesting, when those brown, healthy agouti offspring reproduced, they passed down their coat color! Their babies were also brown.
These results changed our understanding of genetics forever. Even before the Human Genome Project was complete, another research team had already rewritten its biggest assumption. You can control your gene expression. Genes might be your body’s blueprints, but genes do not determine your destiny.
The researchers published their study in 2007, and within the year, interest exploded in what is now known as Epigenetics – the study of the factors that influence our gene expression.
It turns out these factors extend far beyond the prenatal nutrition of agouti mice.
Gene Expression – Out Of The Womb, Into Your Control
At birth, identical twins’ genes are the same, and their gene expression (which genes are turned on and off) is nearly identical. But as twins age, their active and inactive genes begin to diverge. And by adulthood, their gene expression looks nothing alike. Some researchers believe these differences are why one identical twin can develop disease while the other twin remains unaffected.
Even more interestingly, new research suggests that epigenetic differences may explain why one genetically identical twin can bald faster than the other. Here are two more studies here and here.
So what are the factors that influence gene expression and contribute to disease development… or disease prevention?
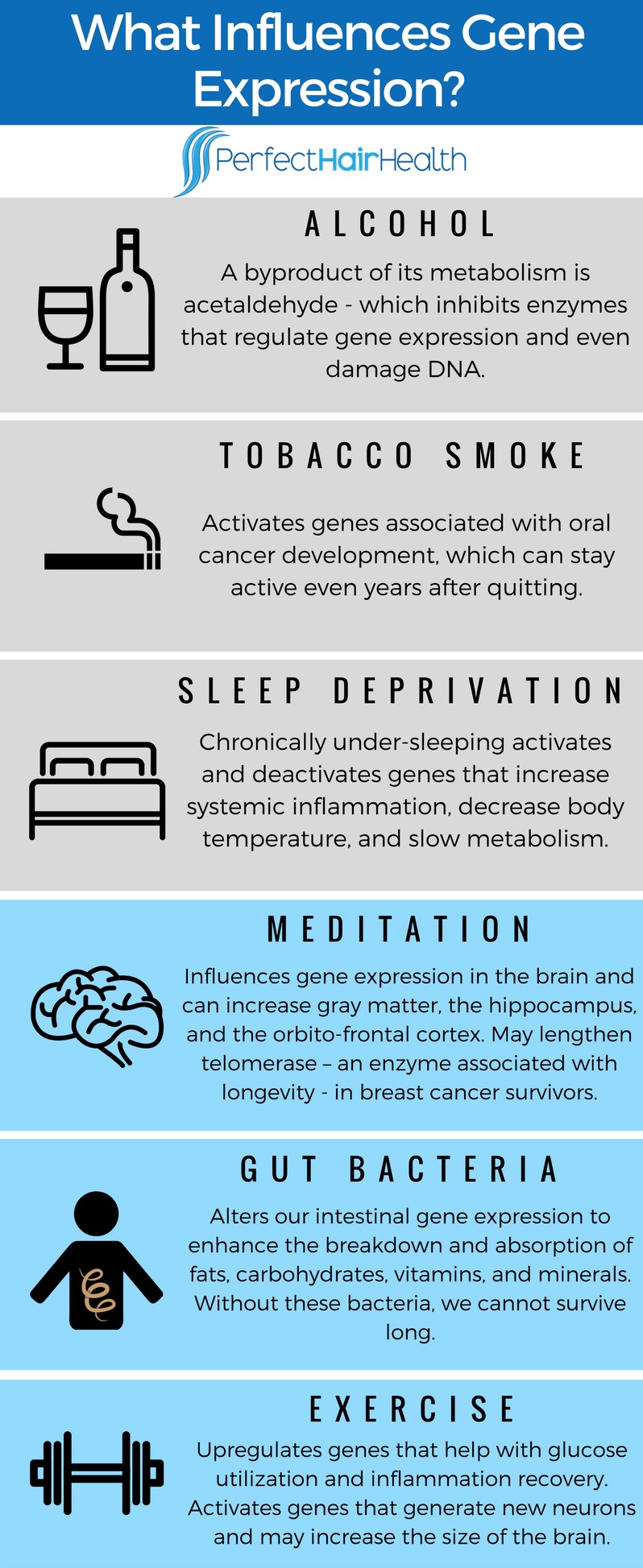
What Influences Gene Expression?
The short answer is everything. Let’s start with the bad:
- Acetaldehyde – a byproduct created when our bodies process alcohol – can inhibit enzymes that regulate gene expression and normal cell function. In some tissues, like the brain and the liver, too much acetaldehyde exposure can even damage DNA.
- Tobacco smoke can activate genes in the mouth that are associated with cancer development. And for some people, these genes stay turned on even years after quitting. This partially explains smoking’s cancer and survival rates.
- Long-term sleep deprivation – through all-nighters or consistently under-sleeping – activates and deactivates genes that control inflammation, thermoregulation, and energy breakdown. The result: increased inflammation, a decreased body temperature, and a slower metabolism.
The list goes on… But now for the good:
- Practicing meditation, mindfulness, and deep focus influences gene expression in our brain, and over time, can grow the brain’s gray matter, hippocampus, and orbito-frontal cortex. It can even lengthen telomerase – an enzyme associated with longevity – in breast cancer survivors.
- Our gut bacteria – the organisms inside our intestines that aren’t human – feed off food we ingest, and then produce enzymes as byproducts. These enzymes create a mutually beneficial relationship with our human cells. They alter our intestinal gene expression to enhance the breakdown and absorption of fats, carbohydrates, vitamins, and minerals. In fact, without these bacteria, we cannot survive long.
- Exercise upregulates (activates) genes in muscle tissues that help with glucose utilization and inflammation. The more you exercise, the stronger this gene expression, the more efficient a muscle becomes at using glucose, the quicker that muscle recovers from inflammation. Moreover, regular exercise upregulates genes that trigger brain stem cells to make new neurons, even growing the size of the brain itself.
Here’s an infographic:
As we can see, the factors influencing our epigenome are extensive. Anything from the air we breathe to the foods we eat to the amount we sleep can change which genes our bodies upregulate and downregulate.
Which brings us to an important question…
Can we influence our gene expression to arrest or even reverse pattern hair loss?
Yes. Researchers have even demonstrated this in identical twin subjects.
Take this study measuring one-year outcomes of genetically identical twins – one using the drug dutasteride (an off-label treatment for hair loss); the other doing nothing for their hair. The one seeking treatment improves their hair loss; the other continues to experience hair loss.
Or take this long-term outlook from two balding twins – one who used dutasteride for years to manage hair loss, the other who hadn’t. The former retained most of their hair; the latter continued to go bald.
In these cases, dutasteride and/or finasteride lowered levels of DHT, a hormone that is causally linked to pattern hair loss. By lowering DHT, these participants most likely decreased the genetic expression of a signaling protein called transforming growth factor beta 1 in dermal papillae cell sites (i.e., the “powerhouse” of the hair follicle). The end-result? A slowing, stopping, or partial reversal of pattern hair loss.
This is just one example of how drugs can influence our own gene expression to improve pattern hair loss. There are many others – including speculation from treatments that aren’t drug-related (like low-level laser therapy or microneedling). But that’s far another article.
For now, what’s important is that gene expression – and our ability to change it – is the basis for what makes hair regrowth possible.
So, the next time your doctor tells you that hair loss is genetic, please don’t feel helpless.
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Perfect Hair Health Team
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
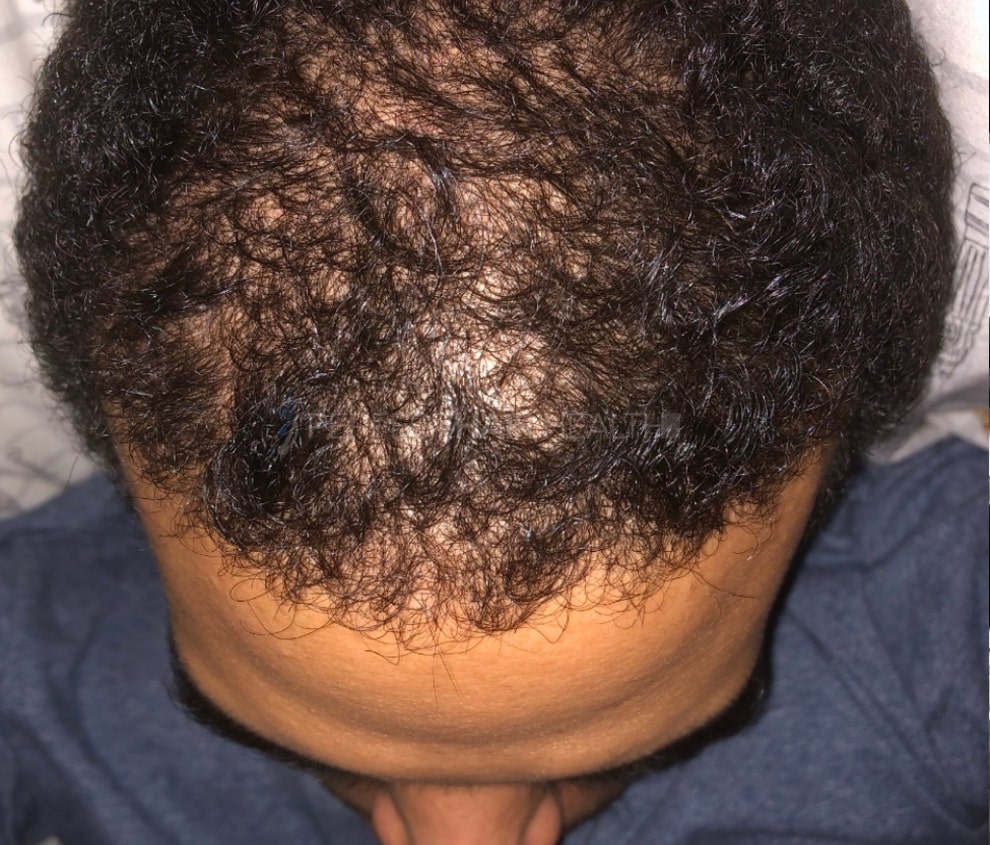
 — RDB, 35, New York, U.S.A.
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
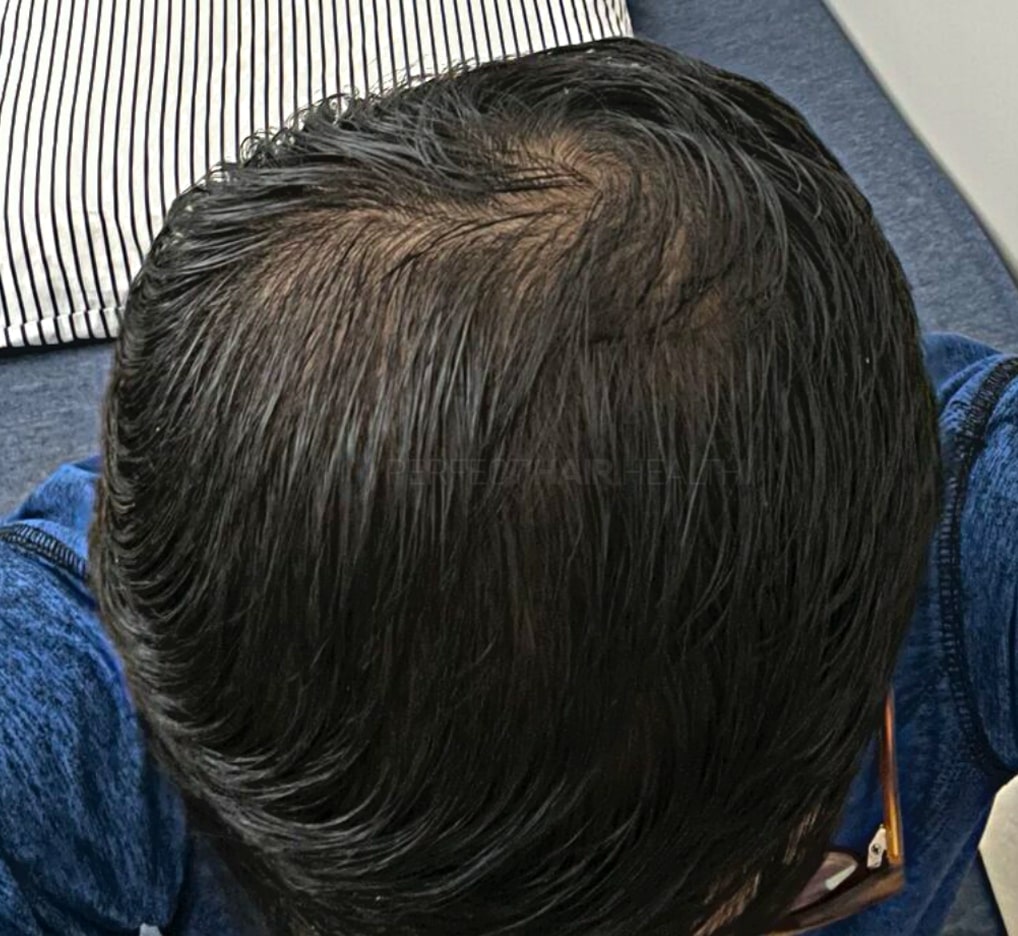 — Aayush, 20’s, Boston, MA
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
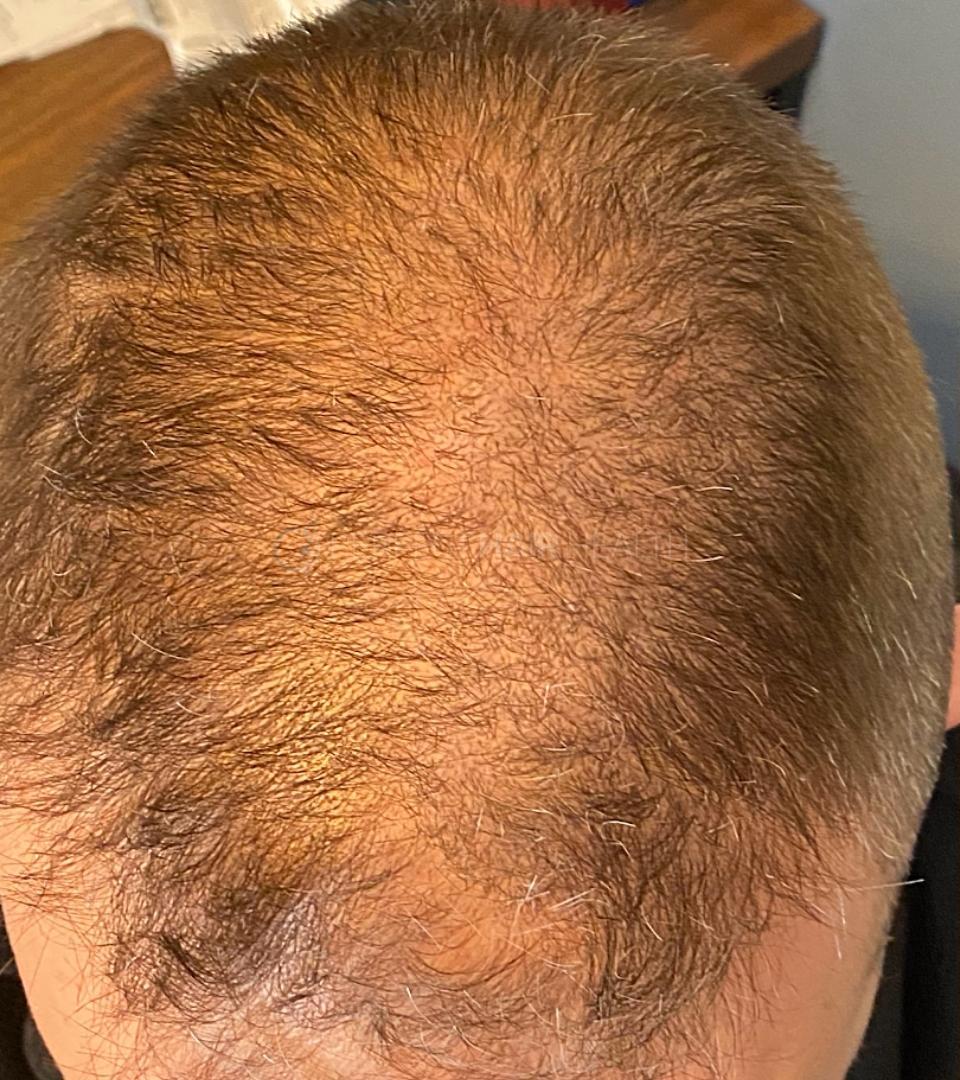
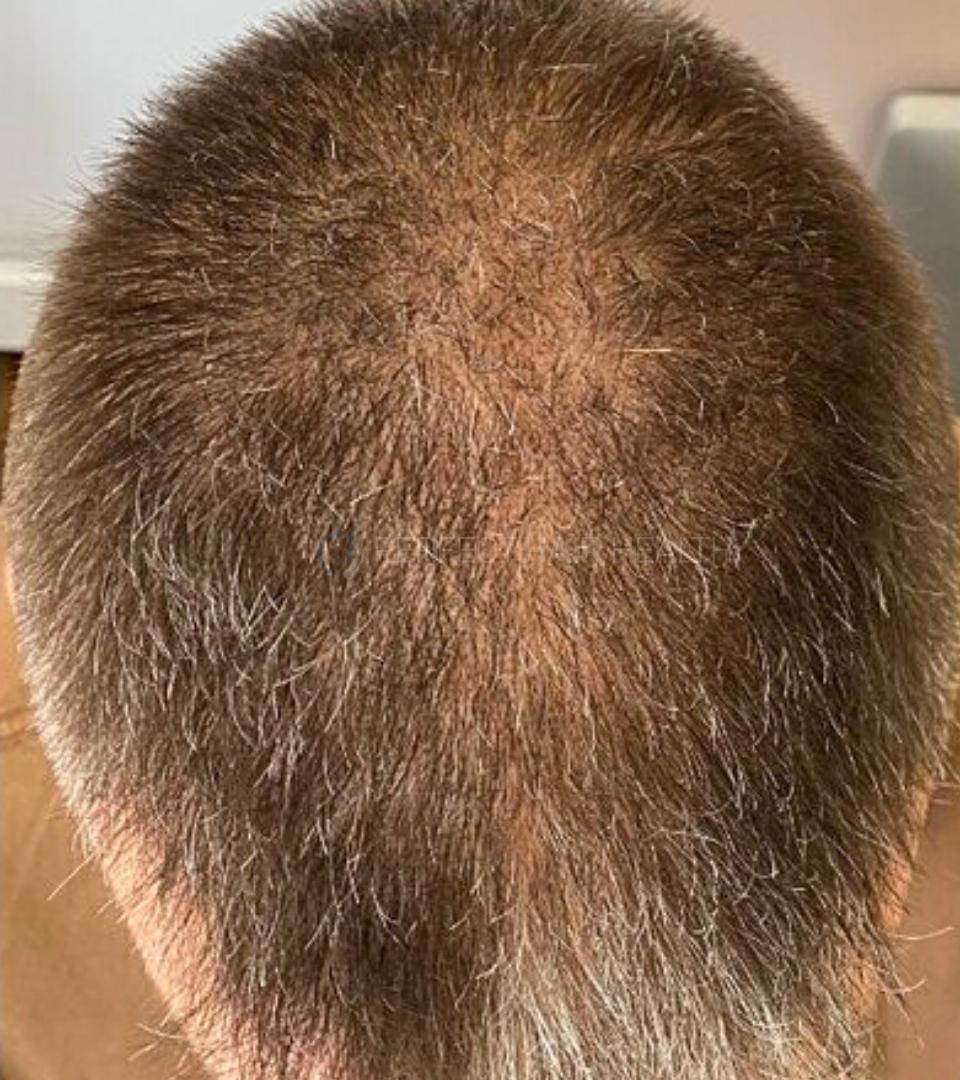 — Douglas, 50’s, Montréal, Canada
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement