- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
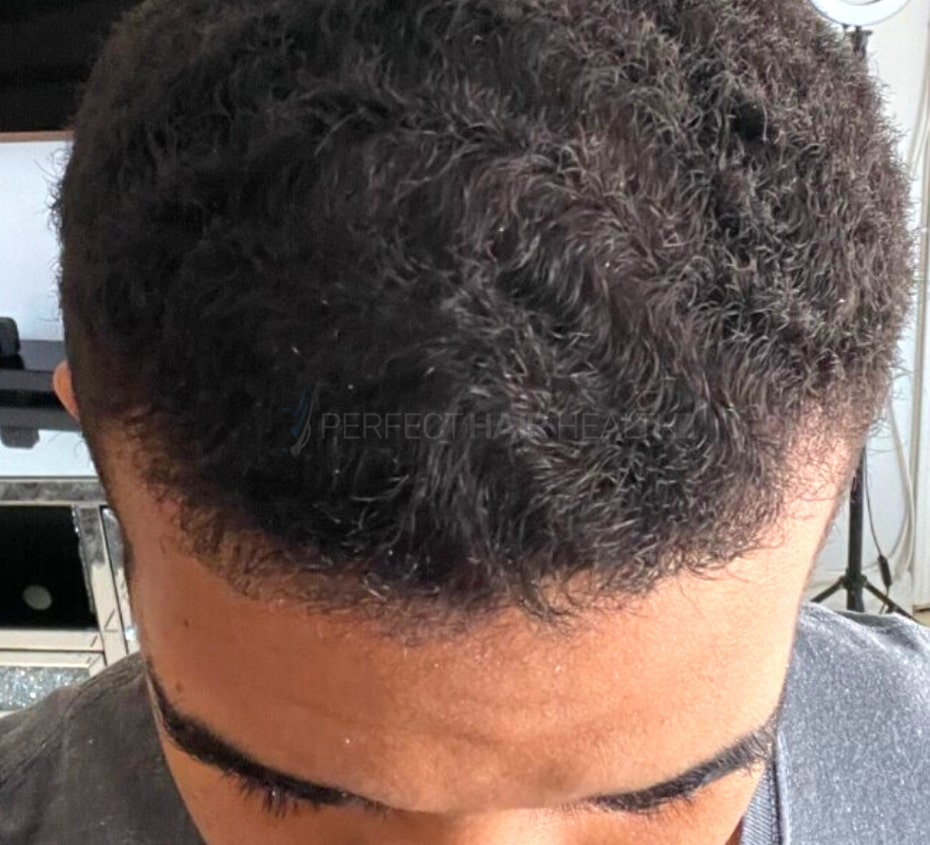
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
 Scroll DownPopular Treatments
Scroll DownPopular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Topical Finasteride Calculator
- Interactive Guide: What Causes Hair Loss?
- Free Guide: Standardized Scalp Massages
- 7-Day Hair Loss Email Course
- Ingredients Database
- Interactive Guide: Hair Loss Disorders
- Treatment Guides
- Product Lab Tests: Purity & Potency
- Evidence Quality Masterclass
More
Articles100+ free articles.
-
Cannabidiol (CBD) Increases Hair Counts By 246%? Not So Fast.
-
Creatine: Does It Worsen Hair Loss? It Depends On The Hair Loss Type.
-
Can Progesterone Improve Hair Regrowth?
-
CRABP2: Can This Gene Predict Regrowth From Retinoids?
-
BTD: Can This Gene Predict Regrowth From Biotin?
-
COL1A1: Can This Gene Predict Regrowth From Collagen Support?
-
2dDR For Hair Loss: What Do We Know So Far About This Sugar?
-
CYP19A1: Can This Gene Predict Regrowth From Hormone Therapy?
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
ArticlesNiostem: Can This Device Stimulate Hair Regrowth? Analyzing Marketing Claims Vs. Reality
First Published Apr 4 2024Last Updated Oct 29 2024Company ReviewsNatural Remedies Researched & Written By:Sarah King, PhD
Researched & Written By:Sarah King, PhD Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor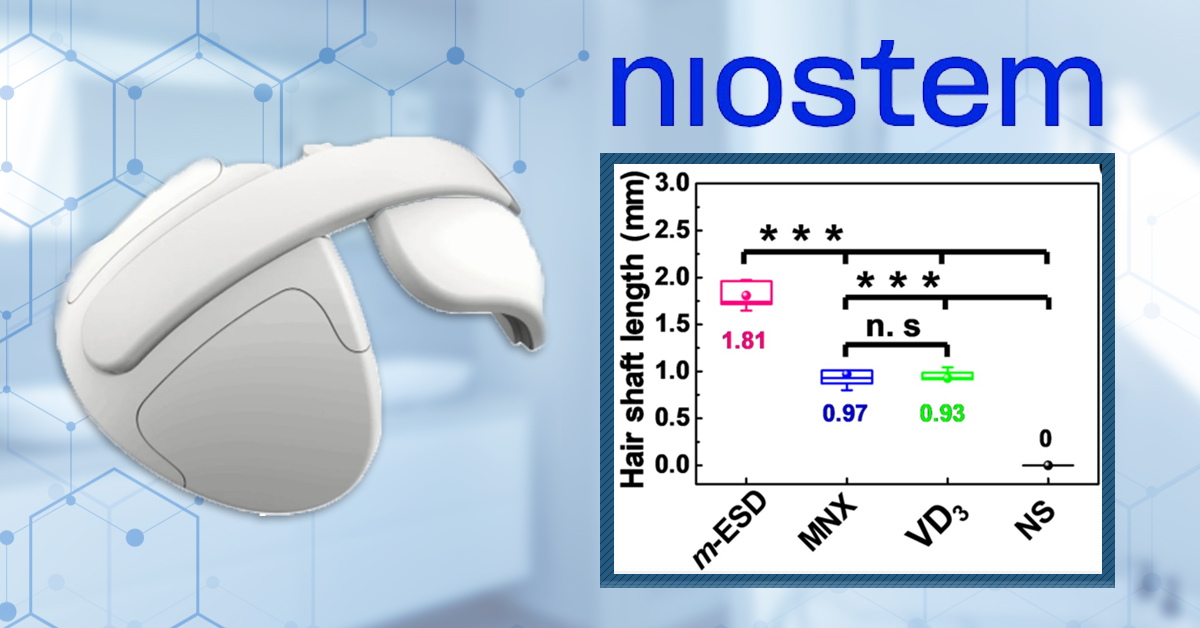
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
Niostem claims their device achieves hair regrowth through electrotrichogenesis & produces better results than finasteride or minoxidil – without “nasty side effects”. Our investigation found many of Niostem’s claims to be unsubstantiated. (1) Niostem has not conducted any comparative clinical studies versus finasteride or minoxidil; their claims stem from scientifically disingenuous comparisons of other studies using different hair counting methodologies & on different hair loss patients. (2) Niostem’s support team revealed their device can produce side effects similar to topical minoxidil: a headache lasting 2-4 days & scalp itchiness. In this article, we review the science on Niostem, make recommendations to the company, & uncover who might be a candidate for this experimental device.
Full Article
Niostem is an at-home wearable device that purportedly uses electrical stimulation to stimulate hair follicle growth. Dr. Chacon-Martinez and Emil Aliev invented the device together and also founded the eponymous company, Niostem. This product is currently in the pre-order phase and will supposedly be available on Indiegogo (a crowdfunding website) in the near future.[1]Niostem, (no date), Niostem. Available at: https://niostem.com/ (Accessed: 26 April 2023
In this article, we will break down some of the claims made on Niostem’s website, and determine if there is any science behind using low-level bioelectrical stimulation to stimulate hair follicle growth (a process termed ‘electrotrichogenesis’) in patients with androgenetic alopecia.
Key Takeaways
- Branding. Niostem is a wearable device intended for home use. The company claims that the device uses low-level electrical stimulation to reactivate hair follicle stem cells and stimulate hair regrowth (also termed electrotrichogenesis). It is targeted at men with androgenetic alopecia.[2]Niostem, (no date), How It Works. Available at: https://niostem.com/pages/how-it-works (Accessed: 25 April 2023
- Unique Selling Point. Niostem claims that its product is different because it is the world’s first device to regrow hair in 6 months.[3]Niostem, (no date), Niostem. Available at: https://niostem.com/ (Accessed: 23 April 2023 The company also states that their low-level bioelectrical stimulation reactivates dormant hair follicle stem cells to restore hair growth and that, instead of meddling with your hormones (presumably referring to pharmaceutical therapies like anti-androgens), they “…reactivate proper cell functionality”.[4]Niostem, (no date). FAQ. How is Niostem Different? Available at: https://niostem.com/pages/faq (Accessed 23 April 2023
- Clinical Support. One 6-month pilot study has been completed by Niostem, comprising 22 men aged between 20-50 that had androgenetic alopecia.[5]Niostem, (no date), FAQs, Does Niostem Work? Available at: https://niostem.com/pages/faq (Accessed: 24 April 2023 The device was used for 30 minutes every day. Niostem claims that this treatment stopped hair loss in 100% of participants, 100% of participants regrew hair, and 19.3% of participants showed an increase in hair density. A consumer perception questionnaire also showed that 75% of participants perceived a positive change in hair (although they don’t mention what kind of positive change, i.e., growth, thickness, quality).
- Concerns.
- The 6-month pilot study was not pre-registered as a trial or published in a peer-reviewed journal – it is only available on their website. We, therefore, have to trust that Niostem is not omitting any results that may not support what they want to show.
- Niostem compares their non-peer-reviewed, in-house results to peer-reviewed papers that investigate other hair loss treatments such as minoxidil and finasteride. They then use these comparisons to conclude that their product is better. This practice is scientifically disingenuous; it’s an apples-to-oranges comparison across studies with participant groups (and hair counting metrics) that cannot be directly compared. It may also open up the company to a truth-in-advertising lawsuit.
- The only study completed was short-term (6 months), so we do not know the potential effects of more long-term use.
- Only 4 of the 22 participants (19.3%) of men in the pilot study saw an increase in hair density. This is arguably a more important parameter than the growth of new hair.
- Evidence Quality. The Niostem Device scored 11/100 for evidence quality, by our metrics.
- Recommendations to Niostem. We recommend that Niostem conduct pre-registered, large-scale, placebo-controlled studies to determine the efficacy and safety of their product. Moreover, Niostem should be careful about making comparisons of their in-house study to other studies published in peer-reviewed journals as there may be differences in study design and parameters that can affect the results. We would also recommend that Niostem make its pilot study fully available to customers so that we can see any potential variations in the results between participants.
What is Niostem?
Niostem, formally known as Mane Biotech, is a company co-founded by Dr. Carlos Chacon-Martinez and Emil Aliev.[6]Mane Biotech, (no date). Meet our Founders. Available at: https://www.manebiotech.com/ (Accessed: 23 April 2023 On their website Dr. Chacon-Martinez is portrayed as an expert in regenerative medicine with expertise in hair stem cells and skin biology. It goes on to say that Dr. Chacon-Martinez completed his Master’s degree at Ghent University in Belgium, and his PhD at the Dresden University of Technology in Germany. He then undertook his post-doctoral post at the Max Planck Institute for Biology of Aging, also in Germany.[7]Niostem, (no date). The Niostem inventor and co-founder. Available at: https://niostem.com/pages/how-it-works (Accessed: 24 April 2023
Other than the founders, there are two other people noted on the site: Prof. Dr. Carien Niessen – a professor in skin biology at the University of Cologne, and Dr Reza Azar a hair surgeon who has authored or co-authored several papers on hair follicle research. So, it sounds like there are people associated with the company who are accomplished in the field of dermatology/skin research which may add to the credibility of the company and its product. However, the roles that Prof. Niessen and Dr Azar have with the company are not made clear.
The Niostem wearable device is the only product offered by the company (Figure 1). According to the website, the device uses low-level bioelectrical stimulation to ‘wake’ follicular stem cells and kick-start the follicle back into the growing stage (anagen) of the hair cycle.[8]Niostem, (no date), How Niostem Works. Available at: https://niostem.com/pages/how-it-works (Accessed: 24 April 2023 The company clearly want to promote itself (and the product) as rooted in scientific research, and reference a pilot study throughout the website – which we will go into below.

Figure 1: Niostem wearable device. Adapted from:[9]Niostem, (no date), Niostem. Available at: https://niostem.com/ (Accessed: 23 April 2023
Who is Niosterm’s Target Customer?
The Niostem device is currently targeted toward men who are over 18, with male-pattern baldness, as the only study for the device was completed in men. The company does state, however, that future work will include tests on women with female pattern hair loss, but that they also believe the effects of the device should be the same regardless of whether men or women use it.[10]Niostem, (no date). FAQ. Can Niostem be used on women? Available at: https://niostem.com/pages/faq (Accessed 23 April 2023
What Niostem Products are Offered?
The only product offered on Niostem’s website is the Niostem wearable stimulation device. However, it is currently only available for pre-order.
How Does Niostem Claim Their Device is Unique?
Niostem claims that their product is the world’s first device to regrow hair in 6 months.[11]Niostem, (no date), Niostem. Available at: https://niostem.com/ (Accessed: 23 April 2023 Furthermore, the company says that the device reactivates dormant stem cells, thereby restoring hair growth. Niostem makes a number of claims on its website (Figure 2), however, one of the bolder claims that Niostem makes is that their device leads to six times more growth than leading drugs (which we will go into below).

Figure 2: Claims made by Niostem. Adapted from:[12]Niostem, (no date), Niostem. Available at: https://niostem.com/ (Accessed: 23 April 2023
Here are some of the other claims made by Niostem, to which we would like to make some counterclaims:
1. Drugs don’t trigger active regrowth
There is an abundance of evidence that hair loss drugs can increase terminal hair counts, which would qualify as “active regrowth”. For example, a review has been published that goes into detail about the effects of finasteride on hair regrowth, with many studies conducting hair counts. [13]Gupta, A.K., Venkataraman, M., Talukder, M., Bamimore, M.A. (2022). Finasteride for hair loss: A review. Journal of Dermatological Treatment. 33(4). 1938-1946. Available at: … Continue reading Moreover, it’s not clear why regrowth needs to be qualified as ‘active’ – surely there can be no other kind of growth?
2. Shampoos are not science-backed
There is evidence that some shampoos, like those containing 2% ketoconazole, have peer-reviewed data supporting their use in the case of AGA and telogen effluvium, which we would argue qualifies as science-backed.[14]Pierard-Franchimont, C., De Doncker, P., Cauwenbergh, G., Pierard, G.E. (1998). Ketoconazole shampoo: effect of long-term use in androgenic alopecia. Dermatology. 196. 474-477. Available at: … Continue reading
3. Drugs don’t trigger cellular regeneration
In androgenic alopecia, there is evidence that the process of hair follicle miniaturization is driven, in part, by the reduction in size of dermal papillae cell clusters – and that this process is caused by both dihydrotestosterone (DHT) and inflammation.[15]English R, Ruiz S. Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia: Are Biopsy Location, Hair Diameter Diversity, and Relative Hair Follicle Miniaturization Partly … Continue reading Some hair loss drugs – like finasteride and dutasteride – therapeutically lower DHT, which then leads to a resizing of dermal papillae cell clusters in subsequent hair cycles. This is, in effect, cellular regeneration of dermal papillae cell clusters caused by finasteride or dutasteride – which we would argue invalidates Niostem’s claims.
What is their pricing?
You can pre-order this product now, with the first batch being delivered in October 2023. At the time of writing, you can get an early-bird discount by being one of the first 500 reservations. It will cost a discounted price of $899, which is 33% off the full price of $1349. However, to secure this, you also need to pay a reservation fee of $29.
Now, let’s have a look at whether the science lives up to the price.
Product Science: Deep Dive
The Niostem wearable device uses low-level electrical impulses that the company claims activate dormant stem cells in the miniaturized hair follicles of someone with pattern baldness. This leads to some differentiating into progenitor cells, and then growing new hair follicles (Figure 3).[16]Niostem, (no date), How Niostem Works. Available at: https://niostem.com/pages/how-it-works (Accessed: 24 April 2023 Below, we will take a look at the normal cycle of hair follicle stem cells vs. what happens to them during androgenetic alopecia and ask, is this a viable therapeutic strategy?

Figure 3: Effect of low-level bioelectrical stimulation on hair regrowth according to Niostem. Adapted from:[17]Niostem, (no date), How Niostem Works. Available at: https://niostem.com/pages/how-it-works (Accessed: 24 April 2023
The Hair Follicle Cycle and Follicular Stem Cells
The hair follicle contains stem cells that reside in a niche called the bulge – this is a region of the hair follicle that is just below the level of the sebaceous glands (Figure 4). The bulge region can also be identified by the expression of several different marker genes.
During the transition between the (catagen) and resting (telogen) stages of the hair follicle cycle, the hair follicle undergoes a period of regression, where many of the cells that make up the structure of the hair follicle die off. The dermal papilla becomes smaller, more condensed, and moves up towards the bulge.[18]Lin, X., Zhu, L., He, J. (2022). Morphogenesis, growth cycle and molecular regulation of hair follicles. Frontiers in Cell and Developmental Biology. 10 Available at: … Continue reading
In the bulge, the hair follicle stem cells are in a stage of dormancy (or quiescence) during the catagen and telogen stages. However, in the telogen stage, a small fraction of these stem cells migrate to just below the bulge and form a condensed ball-like structure. When the hair follicle regresses, the dermal papilla resides just below this ball of stem cells (Figure 3).

Figure 4: The hair follicle cycle. There are three stages of the hair follicle cycle: anagen = growing stage, catagen = transition/regression stage, and telogen = resting stage. In telogen, some stem cells migrate out of the bulge. The dermal papilla is then able to send activating signals that trigger these cells to divide and specialize into matrix progenitor cells. These cells then further specialize into the cells that make up the rest of the hair follicle. This process is continued throughout life in healthy adults. Adapted from:[19]Lee, S.A., Li, K.N., Tumbar, T. (2021). Stem cell-intrinsic mechanisms regulating adult hair follicle homeostasis. Experimental Dermatology. 30(4). 430-447. Available at: … Continue reading
When the hair cycle transitions from the non-growing to the growing stage of the hair follicle cycle, the stem cells that have moved out of the bulge become activated by specific signals sent by the dermal papilla which induces them to undergo rapid growth giving rise to special cells called matrix progenitors. These then go on to form the cells that make up the rest of the hair follicle. The stem cells that remain in the bulge, then undergo a series of cell divisions to renew stem cell stocks and return to dormancy when the hair follicle shifts back to the catagen stage (Figure 5).[20]Lee, S.A., Li, K.N., Tumbar, T. (2021). Stem cell-intrinsic mechanisms regulating adult hair follicle homeostasis. Experimental Dermatology. 30(4). 430-447. Available at: … Continue reading

Figure 5: The hair follicle stem cell cycle. Stem cells reside in the bulge of the hair follicle, and remain in a dormant state throughout the catagen stage. During telogen, a small number of stem cells migrate out of the bulge, where they are subsequently activated by signals originating from the dermal papilla. These cells then undergo a period of rapid growth, giving rise to matrix progenitor cells which will also give rise to the rest of the cells that make up the new hair follicle. Stem cells that remain in the bulge go through a period of cell division during the anagen stage to rebuild stocks of stem cells and then return to dormancy. Adapted from:[21]Lee, S.A., Li, K.N., Tumbar, T. (2021). Stem cell-intrinsic mechanisms regulating adult hair follicle homeostasis. Experimental Dermatology. 30(4). 430-447. Available at: … Continue reading
What Happens to Hair Follicle Stem Cells During Androgenetic Alopecia?
Androgenetic alopecia (AGA) is generally considered to be an androgen-dependent hair loss disorder, characterized by a specific pattern of hair loss in the scalp. The androgens implicated in the emergence of AGA are testosterone and dihydrotestosterone (DHT). Testosterone is converted to DHT by the enzyme 5α-reductase and DHT then goes on to bind to specific androgen receptors.[22]Kinter, K.J., Anekar, A.A. Biochemistry, Dihydrotestosterone. (2023) In:StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: … Continue reading The dermal papilla in the hair follicle expresses androgen receptors, and this expression is increased in androgenetic alopecia alongside an increase in 5α-reductase activity, indicating that androgens exert their effects on the hair follicle through the dermal papilla.[23]Hibberts, N.A., Howell, A.E., Randall, V.A. (1998). Balding hair follicle dermal papilla cells contain higher levels of androgen receptors and higher 5αthan those from the non-balding scalp. Journal … Continue reading [24]Chen, C.L., Huang, W.Y., Wang, E.H.C., Tai, K.Y., Lin, S.J. (2020). Functional complexity of hair follicle stem cell niche and therapeutic targeting of niche dysfunction for hair regeneration. … Continue reading
As mentioned previously, the dermal papilla induces hair follicle stem cells to specialize into the cells that eventually become the new hair follicle. However, in the balding scalp, the ability of the dermal papilla to fulfill its role becomes compromised. This leads to reduced activation of stem cells and subsequent deteriorating hair growth, a shortened growing phase, and a longer non-growing phase. [25]Chen, C.L., Huang, W.Y., Wang, E.H.C., Tai, K.Y., Lin, S.J. (2020). Functional complexity of hair follicle stem cell niche and therapeutic targeting of niche dysfunction for hair regeneration. … Continue reading
Now that we know a little bit about hair follicle stem cells, we can take a look at how electrotrichogenesis supposedly reactivates hair follicle stem cells and whether there is any clinical evidence to support the claims made by Niostem.
What is Electrotrichogenesis?
Electrotrichogenesis is the stimulation of the hair follicle in the scalp by a low-level electric charge. Previous studies have found that it can be effective at accelerating the healing of chronic wounds.[26]Rajendran, S.B., Challen, K., Wright, K.L., Hardy, J.G. (2021). Electrical stimulation to enhance wound healing. Journal of Functional Biomaterials. 12(2). 40. Available at: … Continue reading
But, what about in the hair? Fortunately, some studies are available to help us determine how electrotrichogenesis might help with hair follicle growth.
To determine if electricity could affect hair regrowth, one study designed a motion-activated, wearable electric stimulation device attached to the backs of shaved rats. The device was activated by the head and neck movements of the rats. [27]Yao. G., Jiang, D., Li, J., Kang, L., Chen, S., Long, Y., Wang, Y., Huang, P., Lin, Y., Cai, W., Wang, X. (2019). Self-activated electrical stimulation for effective hair regeneration via a wearable … Continue reading
Firstly, different strengths of the electric field were tested (0 – 5 volts per cm) over 2 weeks (Figure 6), and the researchers claim that a dose of 3 v/cm led to the most notable hair regrowth. Of course, it is very possible that this hair regrowth would have occurred anyway. The researchers say that they did an experiment using a sham device (a device that does not work) as a control, but this appears to only have been done on one rat, so it is difficult to draw any conclusions.

Figure 6: The effect of a range of different electric field strengths on hair growth over 2 weeks. Adapted from:[28]Yao. G., Jiang, D., Li, J., Kang, L., Chen, S., Long, Y., Wang, Y., Huang, P., Lin, Y., Cai, W., Wang, X. (2019). Self-activated electrical stimulation for effective hair regeneration via a wearable … Continue reading
Next, the effect of the wearable device was compared to 5% minoxidil, vitamin D3, and saline as a control. These were applied to small patches on the back of shaved rats. Hair length measurements were taken, alongside photos of the rats after 3 weeks of treatment (Figure 7). Hair length was significantly increased in rats wearing the device compared to the other treatments. Looking at the comparison images, there also did appear to be more hair in the area treated with the device than in the minoxidil or vitamin D3 treatments. Unfortunately, the researchers did not conduct specific counts of growing and non-growing hairs.
More importantly, not all regions of the skin behave the same way. In fact, there is substantial regional diversity in hair growth.[29]Plikus, M.V., Chuong, C.M. (2008). Complex hair cycle domain patterns and regenerative hair waves in living rodents. Journal of Investigative Dermatology 128(5). 1071-1080. Available at: … Continue reading It is possible that the neck skin where the device was located demonstrated intrinsically more hair growth, and that actually the electrical device had no effect at all. Ideally, the researchers would use more animals in order to compare the exact same anatomical regions, or at least only use bilaterally symmetrical treatment sites, which are less likely to show differences in hair growth potential.[30]Plikus, M.V., Chuong, C.M. (2008). Complex hair cycle domain patterns and regenerative hair waves in living rodents. Journal of Investigative Dermatology 128(5). 1071-1080. Available at: … Continue reading
Figure 7: Effect of the wearable electrical stimulation device compared to 5% minoxidil and vitamin D3. (RIGHT): length of hair shaft. (LEFT):

Representative photo of a rat 3 weeks after treatment. Statistical analysis shows p>0.05 *, P<0.01**, P<0.001*** Adapted from:[31]Yao. G., Jiang, D., Li, J., Kang, L., Chen, S., Long, Y., Wang, Y., Huang, P., Lin, Y., Cai, W., Wang, X. (2019). Self-activated electrical stimulation for effective hair regeneration via a wearable … Continue reading
The researchers then conducted the same experiment on 8-week-old genetically nude mice (mice that lack fur development due to deficiency of growth factors) to determine whether it could induce hair follicle formation (Figure 8). A very small improvement in hair growth was seen over the first 12 days, which then disappeared by the 15th day. The researchers attribute this to the hair follicle cycle shifting to the catagen phase, indicating that the device may initiate the entrance into the growing phase of the hair follicle cycle, but not affect the rest of the hair cycle.

Figure 8: Effect of the wearable device on hair regrowth of nude mice over 18 days compared to minoxidil and vitamin D3 treatment. Adapted from:[32]Yao. G., Jiang, D., Li, J., Kang, L., Chen, S., Long, Y., Wang, Y., Huang, P., Lin, Y., Cai, W., Wang, X. (2019). Self-activated electrical stimulation for effective hair regeneration via a wearable … Continue reading
The researcher’s measurements showed a significant increase in hair length in the wearable device-treated areas of the skin compared to the minoxidil or vitamin D-treated areas (Figure 9). Again, the same crucial problems regarding regional differences in hair growth apply here.[33]Plikus, M.V., Chuong, C.M. (2008). Complex hair cycle domain patterns and regenerative hair waves in living rodents. Journal of Investigative Dermatology 128(5). 1071-1080. Available at: … Continue reading
In order to see whether, in principle, electrical stimulation works to promote hair growth, an experiment needs to be conducted where large numbers of animals are treated with the stimulation device versus a sham device that is applied to the same anatomical location. Only then would it be clear whether the bioelectrical device actually does anything. Once this is established, only then would it make sense to make comparisons to other treatments, but again, only between the same regions of skin.

Figure 9: Effect of the wearable device on hair shaft length over 21 days. Adapted from:[34]Yao. G., Jiang, D., Li, J., Kang, L., Chen, S., Long, Y., Wang, Y., Huang, P., Lin, Y., Cai, W., Wang, X. (2019). Self-activated electrical stimulation for effective hair regeneration via a wearable … Continue reading
So, as yet there is little evidence that bioelectrical stimulation can affect hair growth in rats and mice, but what about in humans?
Two studies completed in 1990 and 1992 investigated the efficacy and safety of electrotrichogenesis in humans. The first was a randomized, placebo-controlled comparative controlled study in which 56 men with male pattern baldness, aged between 19-49, were treated with either the electrotrichogenesis treatment (group A) – which contained 30 people – or a control treatment (group B) – which contained 26 people.[35]Maddin, S.W., Bell, P.W., James, J.H.M. (1990). The biological effects of a pulsed electrostatic field with specific reference to hair. Pharmacology and Therapeutics. 29(6). 446-450 Available at: … Continue reading Each participant was seated in a chair, with their head placed under a semispherical hood which was similar to a salon hair dryer. It is unclear however whether there was any contact made between the device and the scalp at all.
Treatments were given once weekly for 36 weeks, except for weeks 1, 2, 17, 18, and 33 where treatments were given twice weekly. Hairs were counted at baseline, week 12, week 24, and at week 36 (Figure 10). While it does look like the treated group showed a higher hair density, the standard deviation was also very high indicating that there was a lot of variation between individual participants, however, according to the statistics carried out, the differences were statistically significant.

Figure 10: Mean change from baseline of hair density after treatment with (A): the electrical stimulation treatment or (B): the control Adapted from:[36]Maddin, S.W., Bell, P.W., James, J.H.M. (1990). The biological effects of a pulsed electrostatic field with specific reference to hair. Pharmacology and Therapeutics. 29(6). 446-450 Available at: … Continue reading
The second study was conducted by the same group and was an extension of the first study.[37]Maddin, S.W. Amara, I., Sollecito, W.A. (1992). Electrotrichogenesis: Further evidence of efficacy and safety on extended use. Pharmacology and Therapeutics. 12. 878-880. Available at: … Continue reading In this study, the subjects from the previous study were asked if they wanted to continue, however, the placebo-treated subjects would cross over into the treated group. 27 subjects in total (13 from the initial treatment group and 14 from the original placebo – now crossover group.
During this extension phase, hairs were counted at weeks 48, 60, and 66 for the cross-over group and weeks 52, 64, and 70 for the treatment group. Compared to the baseline, the treatment group showed a notable improvement in mean terminal hair number by week 36 (the end of the first study) and this continued through to week 70 (Figure 11). The cross-over group showed a moderate increase in hair number over the first 36 weeks (when they would have been the placebo group) but showed a notable increase after switching to the treatment group.
Unfortunately, because we don’t have any raw data, we have no way of knowing if these results are skewed by a few hyper-responders, so we have to trust the data as it is.

Figure 11: Mean terminal hair counts for either the treatment group or the crossover group. (Counts are including the ones taken from the initial 36 weeks – i.e., study 1 – and then continued to week 70). Adapted from: [38]Maddin, S.W. Amara, I., Sollecito, W.A. (1992). Electrotrichogenesis: Further evidence of efficacy and safety on extended use. Pharmacology and Therapeutics. 12. 878-880. Available at: … Continue reading
So, some interesting results, that perhaps show some potential for electrical stimulation in hair growth, however, there are issues with the human studies (including not showing the raw data) that we cannot ignore, Furthermore, as the human studies were completed in a clinic, rather than using an at-home device like Niostem, we cannot be sure that the product would work in the same way or produce the same results. Moreover, although the researchers state that participants who had used any hair regrowth agent in the 6 months previous to the study, we cannot be sure how careful they were about any other factors such as background or medication that might affect hair growth.
The most important thing, however, is finding out if the product actually works, so let’s take a look at the evidence offered by Niostem.
Does the Niostem Device Work as a Hair Loss Treatment?
Niostem has just two examples of before-and-after photos on its website. However, because the hair is clipped back differently in each of these photos it is difficult to say if some of the coverage in the “after” photo is just because they haven’t properly clipped the hair back (Figure 12).

Figure 12: Example of before-and-after photo from the Niostem website. Adapted from:[39]Niostem, (no date). How it works. Available at: https://niostem.com/pages/how-it-works (Accessed: 24 April 2023
Clinical Evidence
The only clinical evidence associated with the Niostem device is a 6-month pilot study completed on 22 men aged between 20-50 with male pattern baldness.[40]Niostem, (no date), FAQs, Does Niostem Work? Available at: https://niostem.com/pages/faq (Accessed: 24 April 2023 As this study has not been published anywhere, we have to rely on the information provided on the website.
The participants used the Niostem device for 30 minutes daily, and their hair was counted over 6 months using a method called trichoscopy. Trichoscopy is the process of taking photos of (ideally) the same area of hair, and then using those photos to measure changes in hair number, or thickness.[41]Jain, N., Doshi, B., Khopkar, U. (2013). International Journal of Trichology. 5(4). 170-178. Available at: https://10.4103/0974-7753.130385 In the case of the Niostem study, a small tattoo was applied to the scalp so that the researchers could photograph the same areas at each check-in.
The results at first glance may look impressive to some. 100% of participants showed a stoppage in hair loss after 6 months, 100% of participants regrew their hair and 19.3% of participants showed an increase in hair density (Figure 13).[42]Niostem, (no date), How it Works. Available at: https://niostem.com/pages/how-it-works (Accessed: 24 April 2023
But looking closer at this, we see some problems. Because we don’t have the full study methods or the raw data, we do not know what the cut-off is for a participant to fall into the “regrew hair” group. It could be one hair or 100 hairs. Unfortunately, because we don’t have all the details of the study, we can’t say, and this makes it difficult to trust the data.
Now, let’s address the hair density number. Hair density usually refers to the number of individual hairs per square inch of the area observed on the scalp. 19.3% of patients showed an increase in hair density which is 4 out of 22 participants. Not a lot.

Figure 13: Niostem pilot study results. Adapted from:[43]Niostem, (no date), How it Works. Available at: https://niostem.com/pages/how-it-works (Accessed: 24 April 2023
This brings us to the next result that is posted on the Niostem website. A comparison of hair density between the Niostem device, minoxidil, and finasteride (Figure 14).[44]Niostem, (no date), How it Works. Available at: https://niostem.com/pages/how-it-works (Accessed: 24 April 2023 Normally, it would be great that a company is testing its product against the current leading treatments for AGA. However, Niostem has not actually conducted any experiments where these treatments are directly compared to the device. Instead, the company has compared its own in-house, non-peer-reviewed findings to peer-reviewed studies that are investigating hair loss treatments. This also leads us to the other problem of the “6x more hair in half the time” claim.[45]Niostem, (no date). Niostem. Available at: https://niostem.com/ (Accessed: 25 April 2024 These kinds of claims cannot really be made when comparing studies that may have different parameters for how hair growth is measured, what is considered to be regrowth, and parameters for participant acceptance onto the study.
Furthermore, knowing that only around 4 people out of 22 in the study showed an increase in hair density, this number may be from one or two hyper-responders to treatment, and not representative of the wider population that might use the device.

Figure 14: Comparison that Niostem makes against minoxidil and finasteride. Adapted from:[46]Niostem, (no date), How it Works. Available at: https://niostem.com/pages/how-it-works (Accessed: 24 April 2023
Niostem also completed a consumer perception questionnaire, however, we don’t know any details other than that 75% of participants perceived a positive change in their hair.
Is the Niostem Device Safe?
Niostem states that its device is completely safe and will lead to no nasty side effects, unlike drugs, transplantation, and laser caps.[47]Niostem, (no date). Niostem. Available at: https://niostem.com/ (Accessed: 25 April 2023 There was no mention of adverse effects on the website, so we sent them a quick message to ask about it (Figure 15) Some may say that headache is a “nasty side effect” even if it only lasts 2-4 days.
One other concern that we have about the Niostem device is that it is recommended to use the product daily for as long as you want to see results. However, there do not appear to have been any long-term studies for low-level bioelectrical stimulation for this product or any other product. Therefore, we can’t say that it is safe to use in the long term.
Should I Use It?
Niostem has stated that if you are in the late stages of androgenetic alopecia then the product may not be for you due to your cells already being dormant for too long.[48]Niostem, (no date), FAQs. Will Niostem work for me? Available at: https://niostem.com/pages/faq (Accessed: 25 April 2023
You may want to try this product if:
- You are ok paying a high price for a product that has very little data associated with it, and very few studies backing the science for it
- You are male in the early stages of androgenetic alopecia
- No other treatment has worked for you
Otherwise, at this stage in the device’s development, we would not recommend this product for use.
Recommendations to the Company
We recommend that Niostem conduct large-scale, long-term, pre-registered trials with placebo controls to determine the efficacy and safety of their product. Additionally, we would recommend that if Niostem wants to compare the efficacy of their product to other treatments, they should complete their own comparative studies, instead of comparing different studies that may have used different parameters to measure hair regrowth. Moreover, we would recommend that Niostem publish the full pilot study so that customers can see if there are wide-ranging effects of this device on the participant (which we suspect is the case).
Final Thoughts
If you are a male with early-stage androgenetic alopecia and no other treatment has worked for you, then you may want to experiment with this product. However, you may also want to reflect on the high price of the device, the disingenuous product comparisons made the Niostem research team, its (current) unavailability, and the lack of recent scientific evidence associated with the electrotrichogenesis as a viable hair loss treatment in humans. Until more research is conducted, and until Niostem changes its marketing practices, we cannot recommend this product.
References[+]
References ↑1 Niostem, (no date), Niostem. Available at: https://niostem.com/ (Accessed: 26 April 2023 ↑2 Niostem, (no date), How It Works. Available at: https://niostem.com/pages/how-it-works (Accessed: 25 April 2023 ↑3, ↑9, ↑11, ↑12 Niostem, (no date), Niostem. Available at: https://niostem.com/ (Accessed: 23 April 2023 ↑4 Niostem, (no date). FAQ. How is Niostem Different? Available at: https://niostem.com/pages/faq (Accessed 23 April 2023 ↑5, ↑40 Niostem, (no date), FAQs, Does Niostem Work? Available at: https://niostem.com/pages/faq (Accessed: 24 April 2023 ↑6 Mane Biotech, (no date). Meet our Founders. Available at: https://www.manebiotech.com/ (Accessed: 23 April 2023 ↑7 Niostem, (no date). The Niostem inventor and co-founder. Available at: https://niostem.com/pages/how-it-works (Accessed: 24 April 2023 ↑8, ↑16, ↑17 Niostem, (no date), How Niostem Works. Available at: https://niostem.com/pages/how-it-works (Accessed: 24 April 2023 ↑10 Niostem, (no date). FAQ. Can Niostem be used on women? Available at: https://niostem.com/pages/faq (Accessed 23 April 2023 ↑13 Gupta, A.K., Venkataraman, M., Talukder, M., Bamimore, M.A. (2022). Finasteride for hair loss: A review. Journal of Dermatological Treatment. 33(4). 1938-1946. Available at: https://doi.org/10.1080/09546634.2021.1959506 ↑14 Pierard-Franchimont, C., De Doncker, P., Cauwenbergh, G., Pierard, G.E. (1998). Ketoconazole shampoo: effect of long-term use in androgenic alopecia. Dermatology. 196. 474-477. Available at: https://doi.org/10.1159/000017954 ↑15 English R, Ruiz S. Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia: Are Biopsy Location, Hair Diameter Diversity, and Relative Hair Follicle Miniaturization Partly to Blame? Clin Cosmet Investig Dermatol. 2021 Apr 7;14:357-365. doi: 10.2147/CCID.S306157. PMID: 33854354; PMCID: PMC8039045. ↑18 Lin, X., Zhu, L., He, J. (2022). Morphogenesis, growth cycle and molecular regulation of hair follicles. Frontiers in Cell and Developmental Biology. 10 Available at: https://doi.org/10.3389/fcell.2022.899095 ↑19, ↑21 Lee, S.A., Li, K.N., Tumbar, T. (2021). Stem cell-intrinsic mechanisms regulating adult hair follicle homeostasis. Experimental Dermatology. 30(4). 430-447. Available at: https://doi.org/10.1111/exd.14251 ↑20 Lee, S.A., Li, K.N., Tumbar, T. (2021). Stem cell-intrinsic mechanisms regulating adult hair follicle homeostasis. Experimental Dermatology. 30(4). 430-447. Available at: https://doi.org/10.1111/exd.14251 ↑22 Kinter, K.J., Anekar, A.A. Biochemistry, Dihydrotestosterone. (2023) In:StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557634 (Accessed: 25 April 2023 ↑23 Hibberts, N.A., Howell, A.E., Randall, V.A. (1998). Balding hair follicle dermal papilla cells contain higher levels of androgen receptors and higher 5αthan those from the non-balding scalp. Journal of Endocrinology. 156, 59-65. Available at: https://doi.org/10.1677/joe.0.1560059 ↑24, ↑25 Chen, C.L., Huang, W.Y., Wang, E.H.C., Tai, K.Y., Lin, S.J. (2020). Functional complexity of hair follicle stem cell niche and therapeutic targeting of niche dysfunction for hair regeneration. Journal of Biomedical Science. 27(43). 1-11. Available at: https://doi.org/10.1186/s12929-020-0624-8 ↑26 Rajendran, S.B., Challen, K., Wright, K.L., Hardy, J.G. (2021). Electrical stimulation to enhance wound healing. Journal of Functional Biomaterials. 12(2). 40. Available at: https://10.3390/jfb12020040 ↑27 Yao. G., Jiang, D., Li, J., Kang, L., Chen, S., Long, Y., Wang, Y., Huang, P., Lin, Y., Cai, W., Wang, X. (2019). Self-activated electrical stimulation for effective hair regeneration via a wearable omnidirectional pulse generator. ACS Nano. 13(11). 12345-12356. Available at: https://doi.org/10.1021/acsnano.9b03912 ↑28, ↑31, ↑32, ↑34 Yao. G., Jiang, D., Li, J., Kang, L., Chen, S., Long, Y., Wang, Y., Huang, P., Lin, Y., Cai, W., Wang, X. (2019). Self-activated electrical stimulation for effective hair regeneration via a wearable omnidirectional pulse generator. ACS Nano. 13(11). 12345-12356. Available at: https://doi.org/10.1021/acsnano.9b03912 ↑29, ↑30, ↑33 Plikus, M.V., Chuong, C.M. (2008). Complex hair cycle domain patterns and regenerative hair waves in living rodents. Journal of Investigative Dermatology 128(5). 1071-1080. Available at: https://doi.org/10.1038/sj.jid.5701180 ↑35 Maddin, S.W., Bell, P.W., James, J.H.M. (1990). The biological effects of a pulsed electrostatic field with specific reference to hair. Pharmacology and Therapeutics. 29(6). 446-450 Available at: https://doi.org/10.1111/j.1365-4362.1990.tb03837.x ↑36 Maddin, S.W., Bell, P.W., James, J.H.M. (1990). The biological effects of a pulsed electrostatic field with specific reference to hair. Pharmacology and Therapeutics. 29(6). 446-450 Available at: https://doi.org/10.1111/j.1365-4362.1990.tb03837.x ↑37 Maddin, S.W. Amara, I., Sollecito, W.A. (1992). Electrotrichogenesis: Further evidence of efficacy and safety on extended use. Pharmacology and Therapeutics. 12. 878-880. Available at: https://doi.org/10.1111/j.1365-4362.1992.tb03550.x. ↑38 Maddin, S.W. Amara, I., Sollecito, W.A. (1992). Electrotrichogenesis: Further evidence of efficacy and safety on extended use. Pharmacology and Therapeutics. 12. 878-880. Available at: https://doi.org/10.1111/j.1365-4362.1992.tb03550.x. ↑39 Niostem, (no date). How it works. Available at: https://niostem.com/pages/how-it-works (Accessed: 24 April 2023 ↑41 Jain, N., Doshi, B., Khopkar, U. (2013). International Journal of Trichology. 5(4). 170-178. Available at: https://10.4103/0974-7753.130385 ↑42, ↑43, ↑44, ↑46 Niostem, (no date), How it Works. Available at: https://niostem.com/pages/how-it-works (Accessed: 24 April 2023 ↑45 Niostem, (no date). Niostem. Available at: https://niostem.com/ (Accessed: 25 April 2024 ↑47 Niostem, (no date). Niostem. Available at: https://niostem.com/ (Accessed: 25 April 2023 ↑48 Niostem, (no date), FAQs. Will Niostem work for me? Available at: https://niostem.com/pages/faq (Accessed: 25 April 2023 Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Sarah King, PhD
Dr. Sarah King is a researcher & writer who holds a BSc in Medical Biology, an MSc in Forensic Biology, and a Ph.D. in Molecular and Cellular Biology. While at university, Dr. King’s research focused on cellular aging and senescence through NAD-dependent signaling – along with research into prostaglandins and their role in hair loss. She is a co-author on several upcoming manuscripts with the Perfect Hair Health team.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
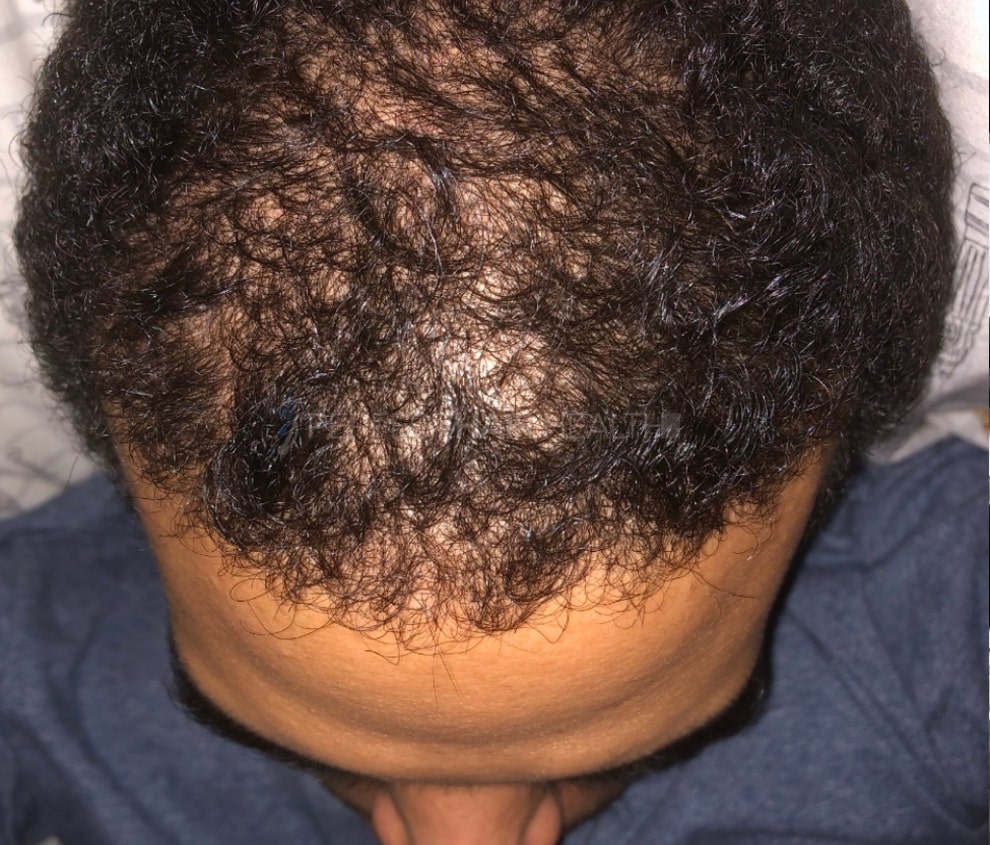
 — RDB, 35, New York, U.S.A.
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
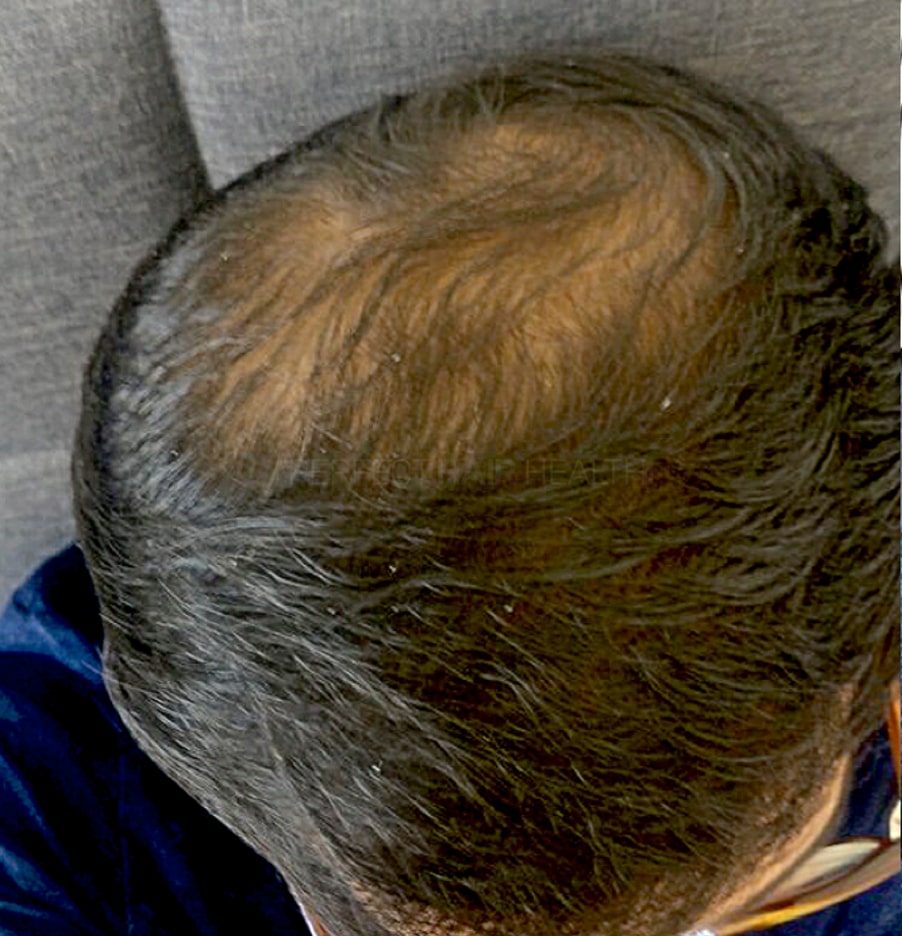
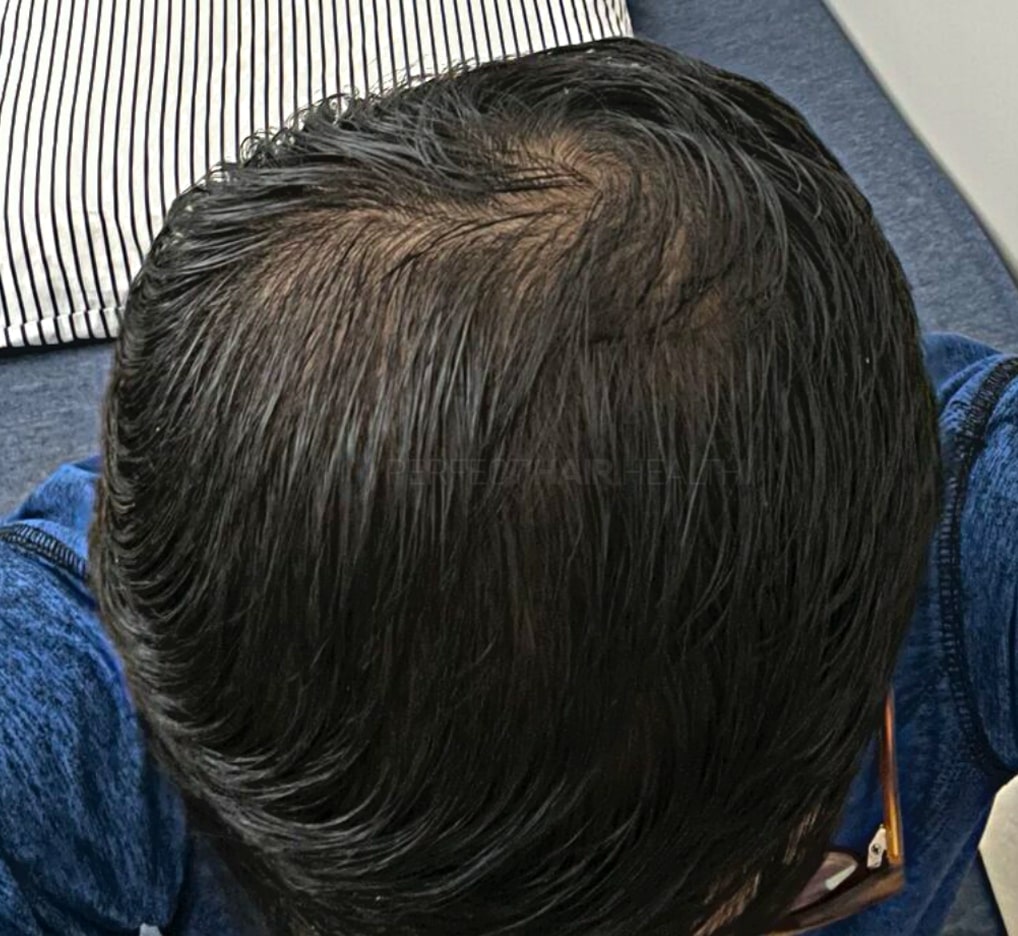 — Aayush, 20’s, Boston, MA
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
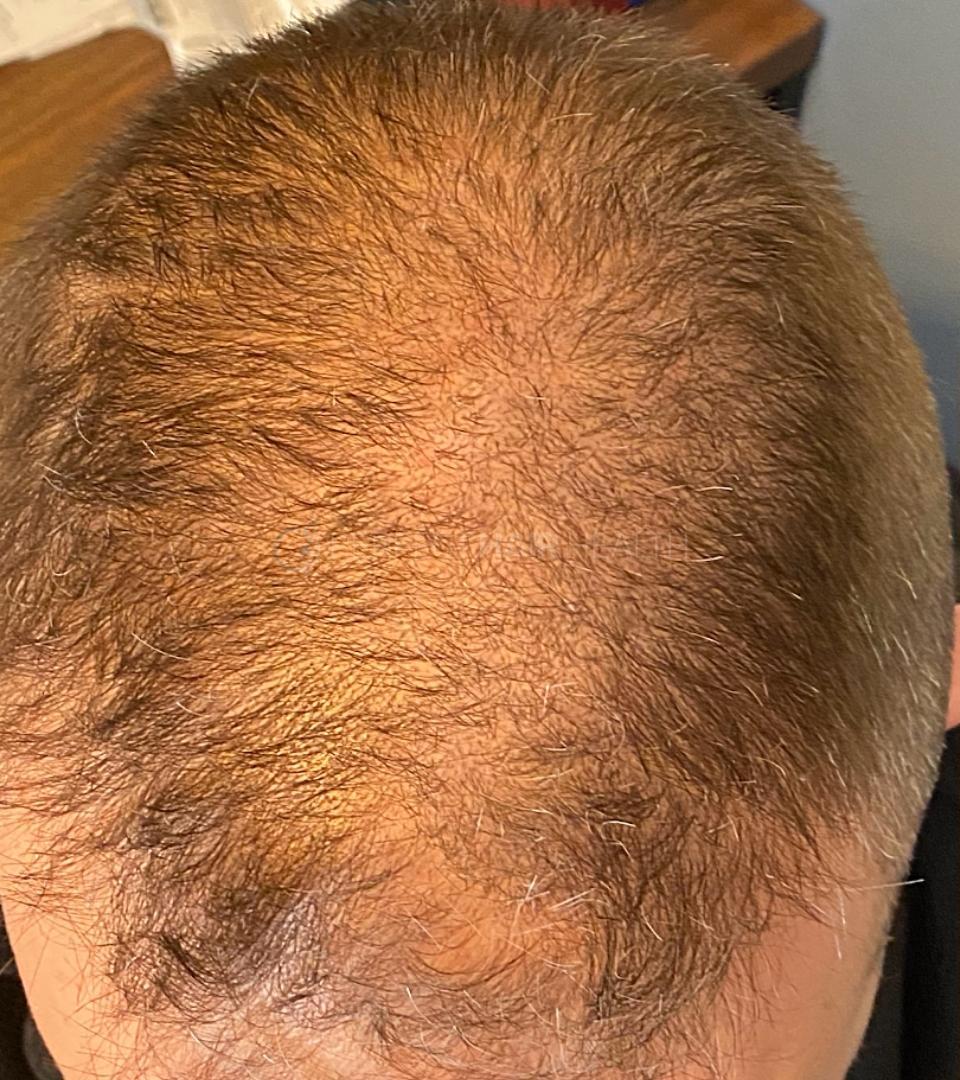
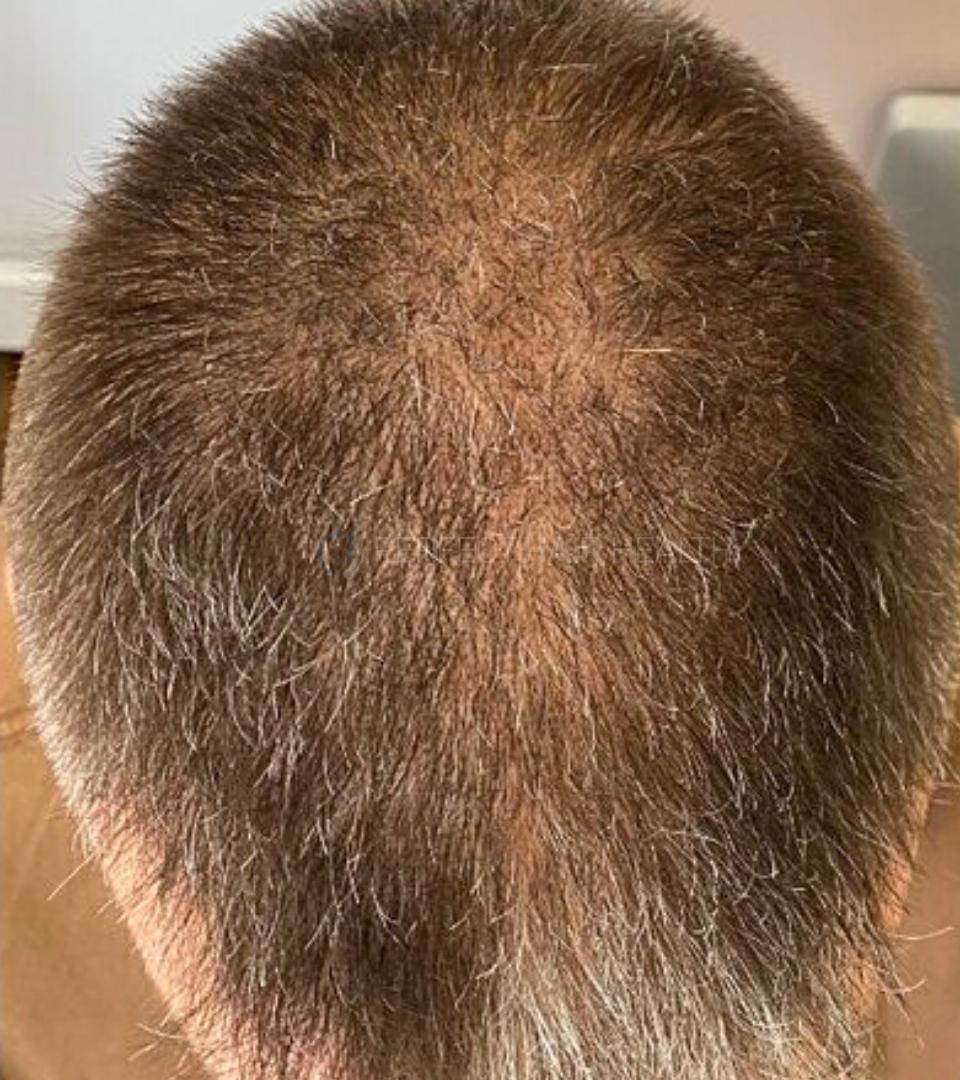 — Douglas, 50’s, Montréal, Canada
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement
