- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
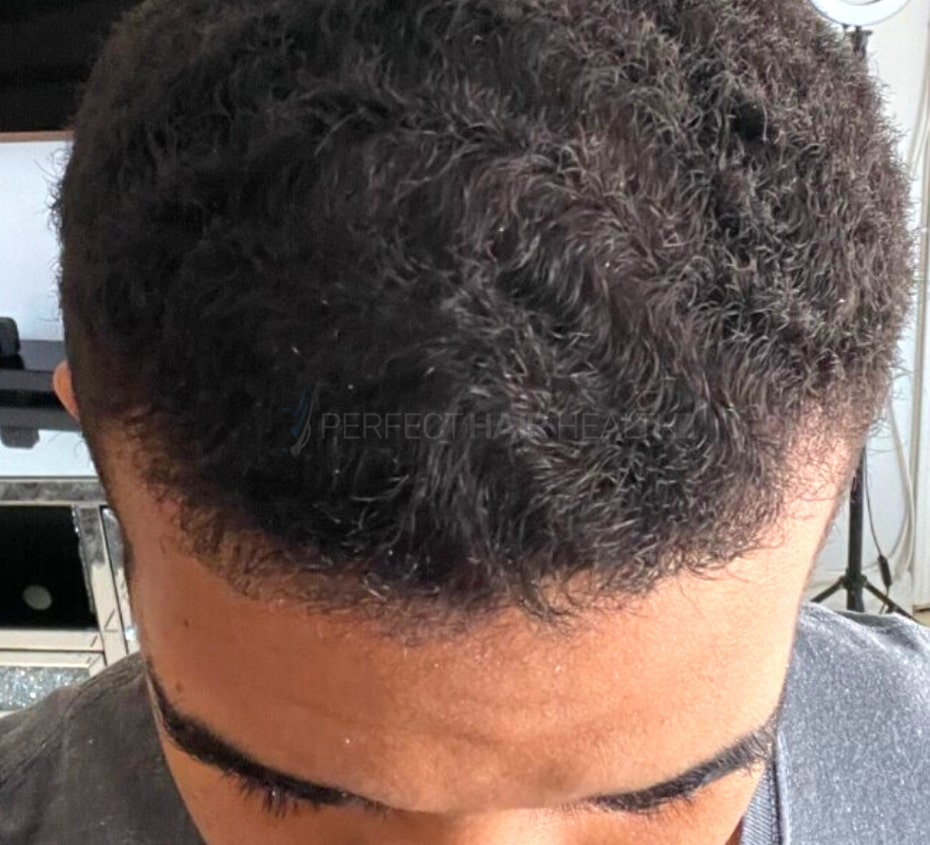
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
 Scroll DownPopular Treatments
Scroll DownPopular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Topical Finasteride Calculator
- Interactive Guide: What Causes Hair Loss?
- Free Guide: Standardized Scalp Massages
- 7-Day Hair Loss Email Course
- Ingredients Database
- Interactive Guide: Hair Loss Disorders
- Treatment Guides
- Product Lab Tests: Purity & Potency
- Evidence Quality Masterclass
More
Articles100+ free articles.
-
Cannabidiol (CBD) Increases Hair Counts By 246%? Not So Fast.
-
Creatine: Does It Worsen Hair Loss? It Depends On The Hair Loss Type.
-
Can Progesterone Improve Hair Regrowth?
-
CRABP2: Can This Gene Predict Regrowth From Retinoids?
-
BTD: Can This Gene Predict Regrowth From Biotin?
-
COL1A1: Can This Gene Predict Regrowth From Collagen Support?
-
2dDR For Hair Loss: What Do We Know So Far About This Sugar?
-
CYP19A1: Can This Gene Predict Regrowth From Hormone Therapy?
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
ArticlesRedensyl, Capixyl, & Procapil (RCP) For Natural Hair Regrowth? (Scientific Review)
First Published Mar 29 2024Last Updated Oct 29 2024Ingredients Researched & Written By:Perfect Hair Health Team
Researched & Written By:Perfect Hair Health Team Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor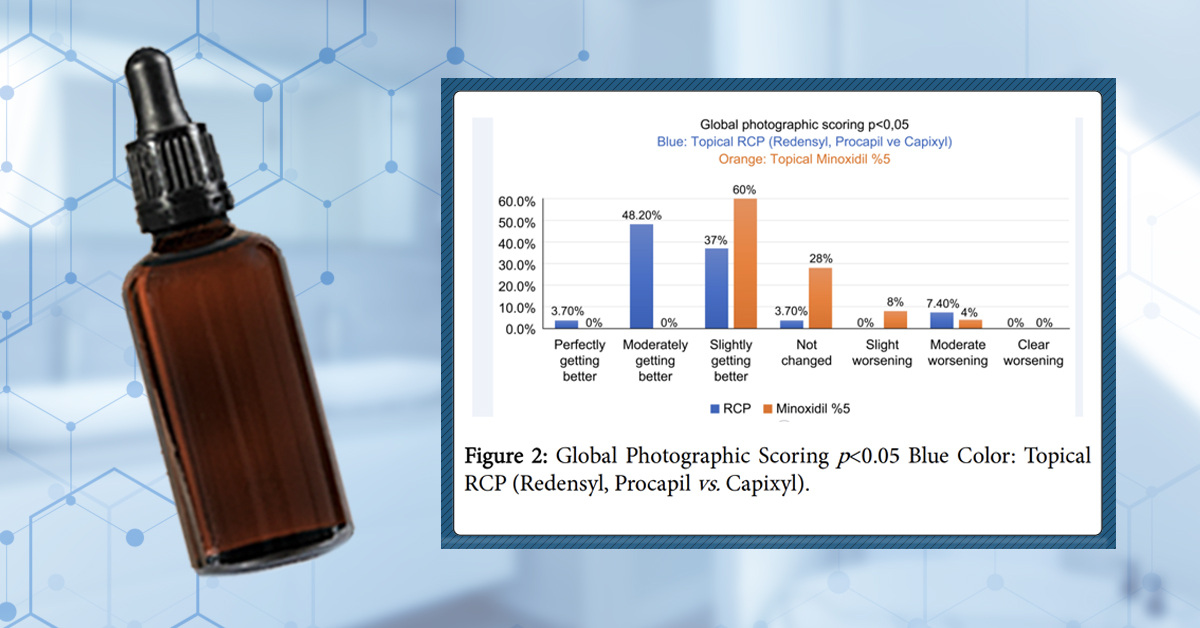
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
Redensyl, Capixyl, & Procapil (RCP) are 3 trademarked blends constituting 12+ natural ingredients marketed for hair regrowth. RCP rose in popularity after a landmark clinical study suggested that topical RCP could regrow hair better than 5% minoxidil. Our investigation into the clinical data on RCP paints a murkier picture. On the one hand, RCP did outperform 5% minoxidil in terms of hair growth parameters. On the other hand, these parameters were subjective endpoints – like “global photograph” & “investigator perceptions” – which are more subject to bias. For each blend – Redensyl, Capixyl, & Procapil – of the clinical studies that do exist to support the individual ingredients, these results are also obfuscated by dose & formulation differences that do not perfectly reflect the ingredients inside RCP. In this review, we dive into everything to know before trying this all-natural product.
Full Article
Product Review: Redensyl, Capixyl & Procapil
Redensyl, Capixyl, and Procapil (RCP) are a set of all-natural products applied topically to fight hair loss. They’re often heralded as alternatives to hair loss drugs like minoxidil – mainly because of the results of a clinical study comparing RCP against 5% minoxidil.
In this product review, we’ll take a look at the science supporting RCP: the mechanisms, studies, and improvements to hair loss versus other hair growth products (both natural and conventional). We’ll evaluate the product claims made by companies selling RCP. Finally, we’ll give our take on the data and reveal whether we do (or don’t) recommend RCP to people looking to fight hair loss even from a natural perspective.
Key Takeaways
- Redensyl, Capixyl and Procapil are three separate products containing an array of flavones, peptides, extracts, and fatty acids. Their manufacturers claim that the ingredients inside these products (and the products themselves) can help lower inflammation and hormones linked to the balding process. Some of these claims are supported by white papers and clinical studies demonstrating that the products (separately and collectively) improve hair parameters.
- Nearly all of the research supporting Redensyl, Capixyl, Procapil, and their combined use all come from research groups hired by the manufacturers selling these products or from companies who’ve patented their combined ingredients. Moreover, most of these studies suffer from small sample sizes and relatively shorter durations (i.e., 4-6 months). All of these things increase the risk of bias in results – where clinical results might not align with reality.
- RCP shouldn’t be considered a frontline therapeutic for androgenic alopecia or any related hair loss disorder. However, since much of the clinical data supporting its ingredients suggest that hair counts tend to plateau for RCP starting around 3 months, hair loss sufferers probably only need to invest up to 6 months to see if the product will benefit their hair.
- Brands selling RCP today do not specify their dilutions of RCP, nor does the one clinical study evaluating RCP against 5% minoxidil. Are these brands giving consumers the right amount of the product for an effect? Unfortunately, we just don’t know.
What are Redensyl, Capixyl, and Procapil?
Redensyl, Capixyl, and Procapil (RCP) are three separately trademarked all-natural products formulated to fight hair loss. More specifically, they’re formulations of polyphenols and/or peptides (i.e., short-chain amino acids). These substances are gaining rapid popularity in natural health circles, even in the absence of any clinical data to support their use for hair (for an example, see our video on Auxano Grow).
RCP rose to popularity after a 2019 clinical study from Turkey found that RCP improved hair counts and hair diameters better than 5% minoxidil in men and women with androgenic alopecia. [1]hilarispublisher.com/…/a-comparative-study-between-topical-5-minoxidil-and-topical-redensyl-capixyl-and-procapil-combination-in-men-with-androg.pdf
Since then, RCP has been featured in dozens of hair loss serums, topicals, and shampoos.
If each product in RCP is trademarked, how is RCP sold together by so many different hair loss companies?
Licensing agreements.
Each of the three products inside RCP is trademarked by separate companies. According to some sleuthing by HairLossCure2020 (which was verified by our team): [2]hairlosscure2020.com/redensyl-capixyl-procapil-and-baicapilhairlosscure2020.com/redensyl-capixyl-procapil-and-baicapil
- Redensyl™ is a trademark of Givaudan (Switzerland).
- Capixyl™ is a trademark of Lucas Meyer Cosmetics (Canada).
- Procapil™ is a trademark of Sederma (France).
Confusingly, the combined formulation of RCP is also patented by a Turkish company under the brand name Procare. While RCP’s 2019 clinical trial does not state any financial disclosures, we suspect Procare is the same company who sponsored that trial. To quote from the study: [3]hilarispublisher.com/…/a-comparative-study-between-topical-5-minoxidil-and-topical-redensyl-capixyl-and-procapil-combination-in-men-with-androg.pdf
“Topical RCP [was] formulated at the Cosmetic Studies Unit R&D Department, Faculty of Pharmacy, Yeditepe University, and all the rights of this RCP combination are reserved by the Turkish Patent Institute under the name Procare.”
In any case, smaller hair loss companies have managed to secure licensing agreements across these companies as a way to feature Redensyl, Capixyl, and Procapil in their own products.
So, what’s inside each of these products? And how might they fight hair loss?
Redensyl
According to its parent company (Givaudan), Redensyl is a topical that targets to improve the “efficiency” of two key parts of the hair follicle: the outer root sheath and the dermal papillae.
These are key areas of interest in hair loss research, mainly because both the outer root sheath and dermal papillae (1) work in concert to control aspects of hair cycling, and (2) express enzymes intimately linked to the balding process – like type II 5-alpha reductase.
To target these regions, Redensyl includes four ingredients:
- Dihydroquercetin-glucoside (DHQG):
- What is it? DHQG is a form of quercetin, a well-known anti-inflammatory plant flavonol.
- Givaudan claims: DHQG is “a stabilized polyphenol which activates the division of hair follicle stem cells, while maintaining their differentiation properties. It protects stem cells from apoptosis (BCL2 activation), and drives them towards the anagen cycle (β-catenin activation), while boosting the metabolism of dermal papilla fibroblasts.”
- EGCG-glucoside (EGCG2):
- What is it? EGCG is another plant flavonol commonly found in green tea, which is able to also reduce inflammation.
- Givaudan claims: EGCG2 is “a stabilized EGCG derivative used to reduce the typical inflammatory state of alopecic scalp (reduction of IL-8), and capture free radicals”
- Glycine:
- What is it? Glycine is an amino acid commonly found in skin and connective tissues such as ligaments, tendons, and cartilage.
- Givaudan claims: Glycine is “a major constituent of hair proteins, mainly keratin associated proteins (KAP), which favors hair growth.”
- Zinc
- What is it? Zinc is an essential trace element, and a cofactor involved in an arrow of processes related to hair cycling.
- Givaudan claims: Zinc is “a very important cofactor for numerous enzymes, favoring the incorporation of cysteine in keratin for a stronger hair shaft.”
Do Givaudan’s claims about Redensyl align with the published data?
Much of Givaudan’s claims from their marketing materials are supported by data that haven’t undergone peer review. In other words, the claims appear supported predominantly by data conducted, collected, analyzed, and published by the company itself – all without the scrutiny of other researchers or hair loss experts.
In fact, inside their marketing materials, the most recent peer-reviewed publication Givaudan cites is from 2000. That’s 22 years ago (i.e., several lifetimes in the world of biological research).
Nonetheless, there is some evidence (from cell cultures and mouse models) that might corroborate some of Givaudan’s claims.
While we couldn’t find any published data on DHGQ, we did find studies on a similar molecule – dihydroquercetin (also called taxifolin) – showing that it could act as an anti-inflammatory and protect against UV radiation in mice. [4]ncbi.nlm.nih.gov/pmc/articles/PMC3435475
Do these anti-inflammatory effects also translate to DHQG, and to human scalps affected by hair loss? We have no idea. Keep in mind that, in most cases, results from cell culture studies and mouse models do not translate to humans with hair loss.
Similarly, there’s a case report on two subjects using EGCG topically and orally (along with three other ingredients in a special delivery vehicle) – each of whom showed significant hair regrowthafter six months. [5]mdpi.com/2079-9284/7/3/65 There’s also a study from the 1970’s showing that gelatin (which contains glycine) might improve hair thickness in those supplementing with it daily. [6]researchgate.net/…/279548216_Effect_of_daily_gelatin_ingestion_on_human_scalp_hair
But these studies are problematic. They’re either conducted on cell cultures and/or mouse models, or they’re conducted in human trials with very small sample sizes, in conjunction with ingredients not featured in Redensyl, and/or with novel delivery vehicles.
Which begs the question…
What’s the real clinical evidence on Redensyl?
In their marketing materials,[7]https://cdn.shopify.com/s/files/1/0319/8073/files/Redensyl_Givaudan_brochure.pdf?v=1617389628Givaudan claims that “Redensyl™ shows outstanding results after 3 months at the clinical level.” They also claim that human subjects testing Redensyl saw “+10,200 hairs in 3 months: better results than one hair transplantation procedure.”
At the outset, these claims are hard to believe. And similar to their mechanistic claims, Givaudan includes no referencesto support the assertion that Redensyl use can lead to 10,200+ new hairs in 3 months.
As part of our process for due diligence, we tried our best to substantiate these claims by conducting a literature search in PubMed. We searched for the individual terms “dihydroquercetin-glucoside” and “redensyl” in relation to hair.
We found a single result:

Is this the clinical study Givaudan is using to substantiate the claims in their marketing materials? It was conducted on humans, and it had evaluation parameters set at the 0-, 3-, and 6-month mark. So, we dug deeper.
“Redensyl” clinical study (2020)
This investigation group sought to determine if a new product formulation – Redensyl – might help fight androgenic alopecia. So, they set up a six-month study to determine if this topical could improve hair loss in men and women with androgenic alopecia.
Specifically, the researchers recruited 44 subjects with androgenic alopecia (AGA) and then randomized them into a treatment and placebo group. At 0, 12, and 24 weeks of treatment, they plucked 40 hairs from the subjects’ heads to measure the ratio of anagen:telogen hairs (i.e., the number of growing versus resting hairs). They also documented hair changes via photographs and patient satisfaction scores.
The results? According to investigators:
- 100% of patients demonstrated either hair loss stabilization, moderate improvement, or great improvement.
- Anagen:telogen ratios (i.e., the ratio of resting versus growing hairs) improved.
- No significant adverse events were reported.
At face value, this appears encouraging. There are just two massive problems.
Problem #1. This study does not corroborate a 10,200+ increase in hair counts
If it isn’t already obvious, this study did not measure hair counts for subjects. Instead, it measured hair-plucking outcomes, photographic changes, and assessments from both the subjects and the investigators.
In other words, we cannot use this study to corroborate the claim that Redensyl improves hair counts by over 10,200 hairs in just 3 months. And that’s just problem #1.
Problem #2. This study wasn’t even conducted on Redensyl
That wasn’t a typo above. The study is on Redenyl, not Redensyl.
Redenyl is a combination of:
- Redensyl® 3%. This is the formulation sold by Givaudan.
- Sepicontrol A5® 4%. According to investigators, “Sepicontrol A5® contains capryloyl glycine, sarcosine and cinnamonum zeylanicum bark extract. It exhibits several actions including 5α reductase activity and antiinflammatory effect that might be beneficial in AGA.”
- Menthol 1.5%. Menthol is a chemical extract from the mint family of plants. In cell cultures, menthol has been shown to have anti-androgenic properties, which may or may not translate to improvements in androgenic alopecia.
If we want to know if Redensyl actually improves hair loss, we cannot test it alongside other ingredients that might fight hair loss. Why? Because if we get a positive result, we’ll never be able to discern how much hair regrowth came from Redensyl versus the other ingredients.
This led us back to our original question: is there any clinical study measuring the ingredients on Redensyl in isolation? According to our research, the answer remains “no”.
But, in a much broader literature search, our team member Pedro did find another study testing Redensyl – albeit alongside a slew of other hair growth-promoting ingredients (like caffeine).
Perhaps this is the study used by Givaudan to support their claim of a 10,200+ increase in hair counts over 3 months?
Redensyl + other ingredients clinical study (2020)
In this study, researchers sought to determine if a topical that included Redensylalongside caffeine, glycine, zinc, and salts might improve hair loss in men with androgenic alopecia. [8]onlinelibrary.wiley.com/….1111/jocd.14158 So, they conducted a double-blinded, placebo-controlled, randomized clinical trial on 62 men with AGA and asked the men to apply the lotion (or placebo) daily for six months. The researchers used phototrichograms to assess changes to hair density and anagen:telogen ratios.
After six months, what were the findings?
- Compared to the placebo group, the treatment group saw a 9% improvement to the ratio of anagen:telogen hairs.
- Compared to the placebo group, there was no statistical change in hair density.
In other words, while Redensyl slightly improved the number of growing versus resting hairs, these changes did not correspond with any statistical improvement to overall hair density versus the group receiving the placebo lotion.
So, not only did this clinical study not demonstrate clinical improvements to hair density versus placebo with Redensyl, but it also had the same problems as the original study: additional ingredients that might also promote hair growth.
Moreover, while reviewing the paper, one of our team members identified claims about Redensyl’s active ingredients that mirrored those inside Givaudan’s marketing materials – in particular, in vitro findings from EGCG and dihydroquercetin in their glucoside forms.
“Polyphenols (Dihydroquercetin-glycoside [DHQG] and Epigallocatechingallate-glucoside [EGCG2]) induced a stimulation of the metabolism of human fibroblast dermal papilla cells, proliferation and anti-apoptotic effect of the outer root sheath cells, and activation of the Wnt/β-catenin pathway.21-23”
This puzzled us, as our initial literature search produced no results to support these claims. Perhaps we missed something? We decided to read the three references to find out.
- The first study does not support any of the above claims. Instead, the study assessed the effects of liposomal DHQG combined with iron and/or copper and how thisaffects the healing process of skin damaged from chemical burns. [9]link.springer.com/…1134/S1990519X12040128
- The second study supports the notion that EGCG – but not EGCG2 – might have a proliferative effect on dermal papillae cells in vitro… but not DHQG. [10]sciencedirect.com/…/pii/S0944711306001383
- The third study is from a journal called, Cosmetics & Toiletries. It isn’t indexed in PubMed. [11]cosmeticsandtoiletries.com/…/making-hair-loss-history-native-polyphenols-to-kick-start-hair-regrowth Usually, a journal isn’t indexed if the journal is (1) predatory, or (2) is of such low-quality that PubMed has chosen to exclude its publications from its platform.
Fascinatingly, that third study matches the same graphs and tables featured in Givaudan’s marketing materials. It features in vitro (i.e., cell cultures), ex vivo (i.e., hair follicles grown outside of the body), and even in vivo (i.e., human) data on 26 male volunteers with AGA.
We could technically refer to this as a third clinical study. So, let’s dive in.
Redensyl clinical study (2017)
At the outset, the study published in Cosmetics & Toiletries is probably the most complete (and promising) data we have on Redensyl. Unfortunately, this isn’t a widely-recognized scientific journal. It’s more of a news source for people working in the cosmetics field.
For an example of the quality of its publications, look no further than its recent post titled, “Expert Opinions: Minimalistic Formulating and Skinimalism”.
“Skinimalism” is a term we’d expect to see in Buzzfeed, not in a scientific journal.
Nonetheless, Givaudan decided to publish their in-house data in this magazine. That data demonstrated, in alignment with their marketing materials, that Redensyl produced proliferative effects on both the outer root sheath and dermal papilla cells of the hair follicle. They also showed, ex vivo, that hair treated with 3% Redensyl grew faster than those treated with 1% minoxidil (but who uses 1% minoxidil?!).

Finally, their study also assessed the use of 3% Redensyl on 26 healthy men with AGA. Like the 2020 investigation group, these men apparently saw a 9% increase in the percent of anagen hairs (i.e., growing hairs), and a 17% decrease in the percent of hairs in telogen (i.e., resting).
The study cited two tables of data to corroborate these findings. But when we clicked into the table links, we were redirected to a series of figures (not tables) that did not show the data.
In fact, we checked the whole article. Despite referencing multiple tables in the results, no tables exist in the paper. We’re not making this up. You can check it for yourself.
In any case, the investigators did provide photographic assessments of men using 3% Redensyl. After almost three months of daily use, here were the results:

Our thoughts on these photos? No significant changes for the featured participants.
Our thoughts on this study? In our eyes, it isn’t really a study…
Instead, it’s a series of data points conducted, collected, and analyzed by the very company with a vested interest in selling the product they’re testing. And, rather than publish that data in a peer-reviewed journal, the data are published in a magazine.
As our team member, Pedro, remarked when reflecting on these circumstances:
“Self-published data is not able to be scrutinized for any faults or misrepresentation. And because of this, products containing Redensyl get away with outlandish claims. In turn, the distributors convince the consumer to buy their products.
This is a worst case scenario of industry-funded science.”
Are there any other clinical studies on Redensyl?
Yes. When combing through all of the references in the above papers, we found one more clinical study featuring Redensyl (and published in a reputable journal). It was conducted in 2020 and it featured 35 women and 10 men with androgenic alopecia and telogen effluvium.
This study showed, over 90 days, that the Redensyl-containing product improved hair counts. But as is the case with two of the earlier studies, this study is not actually on Redensyl; it’s on Redensyl in a nanoencapsulation delivery vehicle, combined with another flavanoid-based product called Baicapil (as well as other hair-promoting ingredients), and used as both a shampoo and leave-in lotion.
If it’s not already obvious, this formulation (and these usage parameters) are not what people using RCP are doing. And regardless, we still cannot parcel out the results of Redensyl from the effects of the other hair loss ingredients.
Redensyl: the bottom line
- Givaudan’s marketing materials claim that Redensyl can increase hair counts by 10,200+ over three months – more than a hair transplant. To our knowledge, there is no published data supporting this.
- Of the published data in reputable journals, studies show (1) no change in hair density versus placebo, (2) an improvement to anagen:telogen ratios, and (3) a very marginal increase to total hair counts with Redensyl – and only when combined alongside other ingredients that promote hair growth (like caffeine).
Capixyl
Capixyl is the second product featured in RCP. According to its manufacturers, Capixyl:
- “Preserves hair follicle stem cells (HFSC) activity”
- “Modulates DHT via 5-α reductase activity inhibition”
- “Stimulates ECM renewal and anchoring proteins synthesis”
- “Decreases pro-inflammatory cytokines”
The makers of Capixyl attribute these effects to the product’s two key ingredients:
- Acetyl-tetrapeptide-3. This is a peptide (i.e., a short-chain amino acid).
- Biochanin A. This is a flavone from red clover extract.

Red clover
How might these ingredients improve hair growth?
Biochanin A mimics testosterone in order to bind to 5-alpha reductase
Biochanin A has a molecular structure similar to testosterone, which allows it to imitate testosterone’s biological roles without eliciting the same effects. This is known as a mimetic. Specifically, biochanin A mimics testosterone by binding to the enzyme 5-alpha reductase. But when it binds, it doesn’t convert itself into DHT. Instead, it remains as a non-androgenic structure. In doing so, it reduces DHT levels.
These effects have been demonstrated in vitro and in mice. However, we don’t yet know if these effects translate to humans with androgenic alopecia. [12]pubmed.ncbi.nlm.nih.gov/7490559, [13]pubmed.ncbi.nlm.nih.gov/23265084
In cell culture studies, Biochanin A also appears to have anti-apoptotic effects. Apoptosis is the process in which cells self-destruct. In androgenic alopecia, this self-destruction occurs more often than in those with healthy hair, and it is believed to accelerate hair cycling (i.e., hair shedding). By inhibiting apoptosis of cell clusters like the dermal papillae, it is believed that shedding can be reduced and that hair can grow longer.
Again, these effects have been demonstrated in vitro and in mice, but have not been confirmed in humans with androgenic alopecia. [14]pubmed.ncbi.nlm.nih.gov/32208265Acetyl-Tetrapeptide-3 may support dermal papillae cells
Unfortunately, there isn’t significant research on acetyl-tetrapeptide-3 and its effects on hair growth. In our experience, this is often the case for most peptides popular in the hair loss space. Keep in mind there are tens of thousands of peptides in existence, each with varying effects in petri dishes. That makes it easy for marketers to pick one that is lesser-researched and start to study / promote it – even when the evidence is relatively shaky.
In any case, white papers from the makers of Capixyl suggest that acetyl-tetrapeptide-3, at least in petri dishes, might help support dermal papillae cells.
Does Capixyl improve androgenic alopecia?
Capixyl’s manufacturers state their product leads to “fuller, thicker and healthier hair and eye lashes”, and “helps reduce inflammation in the scalp”. Is this true in people with androgenic alopecia?
Some clinical studies suggest yes – with caveats.
Like Redensyl, much of the human evidence supporting Capixyl comes directly from research teams working with the manufacturers who sell the product. The clinical studies are also small and run less than a year – all factors that can increase the risk of false-positive results.
Nonetheless, there is still some clinical evidence supporting Capixyl in peer-reviewed journals. We’ll dive into these studies below.
Capixyl: Clinical Study #1
In a 24-week randomized, blinded study on 34 men and women with androgenic alopecia, Capixyl mixed with ginseng was tested against 3% minoxidil for its effects on hair loss – such as changes to terminal hair counts and hair density. [15]ncbi.nlm.nih.gov/pmc/articles/PMC7840088
Overall, the results were encouraging. Capixyl performed similarly to 3% minoxidil in terms of improving terminal hair counts, self-assessments, photographic assessments, and hair mass index.

Pictures A-C are from baseline, 12 weeks and 24 weeks of Capixyl use. Pictures D-F are from baseline, 12 weeks and 24 weeks of 3% minoxidil use.White paper showing phototrichographic results of Capixyl

Having said that, these results need to be caveated with the study’s limitations:
- The study has a very small sample size (i.e., 17 people in the Capixyl group), thus introducing the possibility of statistical noise and false positives.
- Capixyl was combined with ginseng. As such, we don’t know how much of these effects are coming from the Capixyl or the ginseng.
- Men are advised to use 5% minoxidil – not 3% minoxidil – for pattern hair loss. Minoxidil’s effectiveness increases with the dose up through 5%. Therefore, it’s likely that Capixyl would’ve underperformed against 5% minoxidil.
Capixyl: Clinical Study #2
In a second clinical study using only capixyl, 30 participants with androgenic alopecia tested a 5% capixyl topical over four months. [16]researchgate.net/…/235755975_A_new_strategy_to_modulate_alopecia_using_a_combination_of_two_specific_and_unique_ingredients They were screened for any other potential contributions to hair loss such as anemia or hypothyroidism, and the investigators randomized participants into a placebo group and a treatment group (i.e., 5% capixyl).
The investigators also conducted preclinical research (i.e., cell culture tests) to include as part of the trial. These showed that acetyl-tetrapeptide-3 improved the number of fibroblasts in dermal papillae cells, as well as laminins (a type of protein found on the scalp that controls interactions with the skin, the membrane, the dermal papilla, and more).
Fibroblasts can differentiate into myofibroblasts, and these are the culprit behind the fibrosis on the scalp. However, this seems to only occur during times of stress signal where certain pro-inflammatory signalling molecules by the body are released. Under normal conditions, fibroblasts may actually support the integrity of the overall scalp environment.

The graph on the left shows that with Acetyl-tetrapeptide-3, both type III collagen and laminin were more clearly seen in fluorescence microscopy. And based on the intensity, the graph on the right clarifies by how much more they were improved. Type III collagen production improved by 65% and laminins by 285%.
This study did demonstrate improvements to hair parameters in the treatment group. Having said that, many of the results did not reach statistical significance. Specifically, in the treatment group the following results were noticed:
- Non-significant increases in hair density after four months. The placebo group had similar anagen hair density, which made their finding not significant.
- The number of hair follicles increased in the anagen phase of the treatment group by 13%. The control group actually saw a decline in hairs within the anagen phase by -2%. This is expected since four months of no therapy for AGA was given.
- Similarly, hairs in their telogen phase decreased by -29% in the treatment group. The control group saw a rise of overall hairs in telogen phase by +23%.
- When compared to baseline, overall the treatment group saw a significant increase in Anagen to Telogen ratios by 46%. While the placebo group saw a significant decrease in Anagen to Telogen ratio by -33%.
These results suggest that when compared to a placebo, capixyl did appear to help support hair growth and increase the overall ratio of hairs in their growth stage. But again, the results were not significant enough that they would likely create a meaningful difference. Moreover, this study was conducted by researchers working for the manufacturers of capixyl. As such, we need to interpret the findings with a degree of caution.
Capixyl: the bottom line
- Capixyl boasts two clinical studies showing that 5% capixyl, applied daily, may improve hair parameters in men and women with pattern hair loss.
- While these results are encouraging, it’s also worth mentioning that these clinical studies were conducted by researchers hired by the manufacturers of the product. And, like Redensyl, the sample sizes were small and the studies ran less than one yearl – thus increasing risks of false-positive results.
Procapil
Procapil is the final product featured inside RCP. Similar to capixyl, procapil utilizes certain peptides and plant-derived compounds in an effort to reduce DHT and improve scalp health.
The ingredients in procapil include:
- Oleanolic acid. This is a fatty acid derived from olives.
- Apigenin. This is a flavone that is often derived from oranges.
- Glycine-histidine-lysine tripeptide. This is a peptide.
The makers of procapil claim their product helps reduce DHT and support metalloproteinase activity. [17]crodapersonalcare.com/…/technical-library In turn, the hope is that these effects may stabilize the collagenous membrane surrounding hair follicles and the skin.
So, how do these claims stack up to the clinical evidence?
Unfortunately, we don’t know.
To our knowledge, procapil has not yet been evaluated in a single study, at least outside of the trio combination of Procapil, Capixyl and Redensyl (which we’ll review soon). But the active ingredients in Procapil have been evaluated in cell cultures and animal models.
Here’s what we found.
Oleanolic Acid
In cell culture studies, oleanolic acid has been shown to be anti-carcinogenic, anti-inflammatory, and antimicrobial. While these signaling cascades are all important for maintaining a healthy scalp, we can’t extrapolate data on cell cultures to outcomes in humans with androgenic alopecia. So, there’s not much here that we can say.
Apigenin
According to in vitro data (and animal models), apigenin might increase vasodilation and support the amount of blood supply to hair follicles. But again, according to our literature searches, there’s not much here we can say as to whether these results translate to humans with androgenic alopecia.
Apigenin may act as an antimicrobial by generating reactive nitrogen species. [18]pubmed.ncbi.nlm.nih.gov/32845547 One such species is nitric oxide. Since nitric oxide is a supportive gas in vasodilation, it is possible that by generating mild amounts of reactive nitrogen species, increases in blood supply occur. This is very similar to the mechanisms speculated to explain the effects of hair growth from low-level laser therapy.
However, what has not been demonstrated in this mechanism is if this increase in blood supply reaches hair follicles and more importantly, if the reactive nitrogen species causes damage to tissues.
Glycine-Histidine-Lysine Tripeptide
Glycine-histidine-lysine tripeptide (GHL) is a peptide that continues to gain popularity in hair loss products – even with little evidence supporting its mechanisms or effects.
In one in vitro study testing a peptide similar to GHL (called GHK – see our mentions of Auxano Grow here), GHK was shown to support the release of trophic factors like vascular endothelial growth factor, a growth peptide involved in healing the scalp after microneedling. [19]pubmed.ncbi.nlm.nih.gov/24468583 But we don’t know how this ingredient (by itself) affects humans with androgenic alopecia. Again, that doesn’t mean that it won’t work in humans; it just means we don’t know.
Now that we’ve covered each of the products inside RCP, it’s time to move onto how they fare together in the same hair loss topical.
The Clinical Study: Redensyl, Capixyl, & Procapil
While all of these studies on redensyl, capixyl, and procapil are interesting, none of them garnered significant interest from hair loss sufferers until 2019 – when a clinical study out of Turkey combined all three ingredients together, tested them against 5% minoxidil, and found that RCP not only showed favorable results, but also outperformed 5% minoxidil in several hair growth endpoints. [20]hilarispublisher.com/…/a-comparative-study-between-topical-5-minoxidil-and-topical-redensyl-capixyl-and-procapil-combination-in-men-with-androg.pdf

This single study is what put RCP “on the map” of hair loss sufferers, and it’s likely what has enabled companies like The Ordinary and Reviv to market these products together in their own hair loss serums.
In the study, 120 people were randomized into two groups – the RCP group or the 5% minoxidil group – and then asked to apply 1 mL twice-daily of their respective products to the scalp. Six months later, final hair counts were assessed (as well as investigator and participant surveys) along with photographs to determine visual changes to hair density.
Overall, the results indicate that RCP outperformed 5% minoxidil on nearly every metric: investigator, participant, and photographic assessments. Unfortunately, hair count analyses were not conducted, but the overall results were impressive (which is, again, why RCP has gained so much popularity online).

Photographic assessment from the RCP clinical study, summarized in a table
Even still, we do have some concerns about the study:
- This study doesn’t specify the dilution of RCP. In other words, we don’t know what percent dilution of RCP was used in this study, and if that percent dilution aligns with what companies like The Ordinary sell to people in their serums. Keep in mind that studies on Redensyl and Capixyl as standalone products used 3% and 5% formulations, respectively. If we assumed Procapil got a 5% dilution, then we’d expect a total RCP dilution ratio of 13-15%. Is that the dilution used by the RCP formulation in this study (i.e., Procare)? Is this the dilution ratio sold to consumers in The Ordinary? We just don’t know.
- Company-funded research. Again, the research supporting RCP and its standalone ingredients all come from companies selling the product. This isn’t necessarily the end of the world, but it does give us pause to outright recommend the product when there haven’t been any replication studies.
- No hard hair count measurements. Having said that, the subjective assessments from participants (and investigators) do suggest improvements, at least in our eyes.
Our take on the data
Based on all of the evidence, there are several clinical trials supporting the use of Redensyl and Capixyl – and mechanistic evidence supporting the use of Procapil – for the treatment of androgenic alopecia. There’s also evidence that an unknown dilution of RCP can improve hair assessments better than 5% minoxidil.
Having said that, all of this research (to our knowledge) was conducted and funded by companies who developed each of those products, or held patents on their combination. Moreover, most of the studies had very small sample sizes, and some of these studies weren’t even submitted for peer-review. Rather, they were released as white papers from the manufacturers themselves.
Nonetheless, we feel that for people who are looking to fight hair loss naturally, RCP has some merit for testing – at least for those who cannot tolerate FDA-approved drugs or who have zero interest in ever trying them.
While we don’t yet know if these dilutions match those in RCP’s infamous clinical trial, here are our recommendations for testing this product should you choose to try it:
- Test for 4-6 months. According to the clinical data on Capixyl (which we suspect to have the strongest clinical support of any of the ingredients), hair count improvements tended to plateau around month 3 – suggesting we might be able to expect the same from RCP. That means that 4-6 months should give us enough time to see if these new hairs grow out to improve our perceptions of density.
- Use twice daily. The clinical study on RCP called for two applications daily: 1 mL in the morning, and 1 mL in the evening. Try to emulate this as best as possible, and if you miss a session, double up on the following application.
- Evaluate your progress. If things are improving by month 6, great! If not, it’s probably time to drop RCP & end your experiment.
References[+]
References ↑1, ↑3, ↑20 hilarispublisher.com/…/a-comparative-study-between-topical-5-minoxidil-and-topical-redensyl-capixyl-and-procapil-combination-in-men-with-androg.pdf ↑2 hairlosscure2020.com/redensyl-capixyl-procapil-and-baicapilhairlosscure2020.com/redensyl-capixyl-procapil-and-baicapil ↑4 ncbi.nlm.nih.gov/pmc/articles/PMC3435475 ↑5 mdpi.com/2079-9284/7/3/65 ↑6 researchgate.net/…/279548216_Effect_of_daily_gelatin_ingestion_on_human_scalp_hair ↑7 https://cdn.shopify.com/s/files/1/0319/8073/files/Redensyl_Givaudan_brochure.pdf?v=1617389628 ↑8 onlinelibrary.wiley.com/….1111/jocd.14158 ↑9 link.springer.com/…1134/S1990519X12040128 ↑10 sciencedirect.com/…/pii/S0944711306001383 ↑11 cosmeticsandtoiletries.com/…/making-hair-loss-history-native-polyphenols-to-kick-start-hair-regrowth ↑12 pubmed.ncbi.nlm.nih.gov/7490559 ↑13 pubmed.ncbi.nlm.nih.gov/23265084 ↑14 pubmed.ncbi.nlm.nih.gov/32208265 ↑15 ncbi.nlm.nih.gov/pmc/articles/PMC7840088 ↑16 researchgate.net/…/235755975_A_new_strategy_to_modulate_alopecia_using_a_combination_of_two_specific_and_unique_ingredients ↑17 crodapersonalcare.com/…/technical-library ↑18 pubmed.ncbi.nlm.nih.gov/32845547 ↑19 pubmed.ncbi.nlm.nih.gov/24468583 Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Perfect Hair Health Team
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
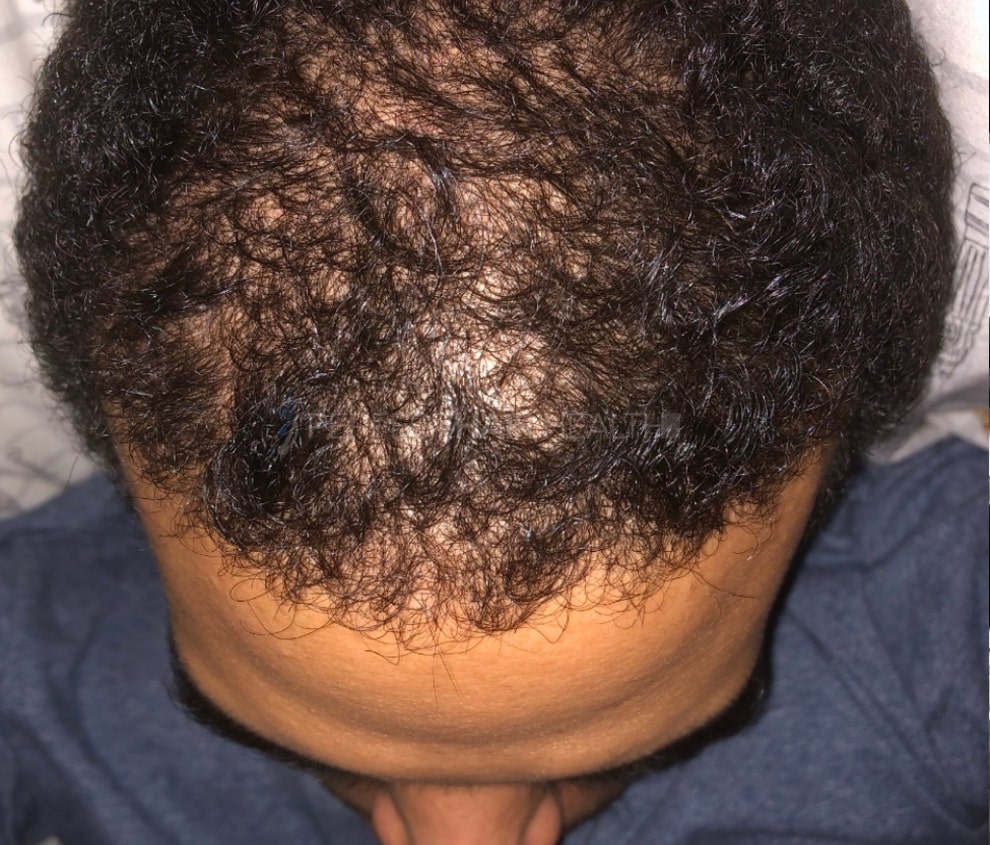
 — RDB, 35, New York, U.S.A.
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
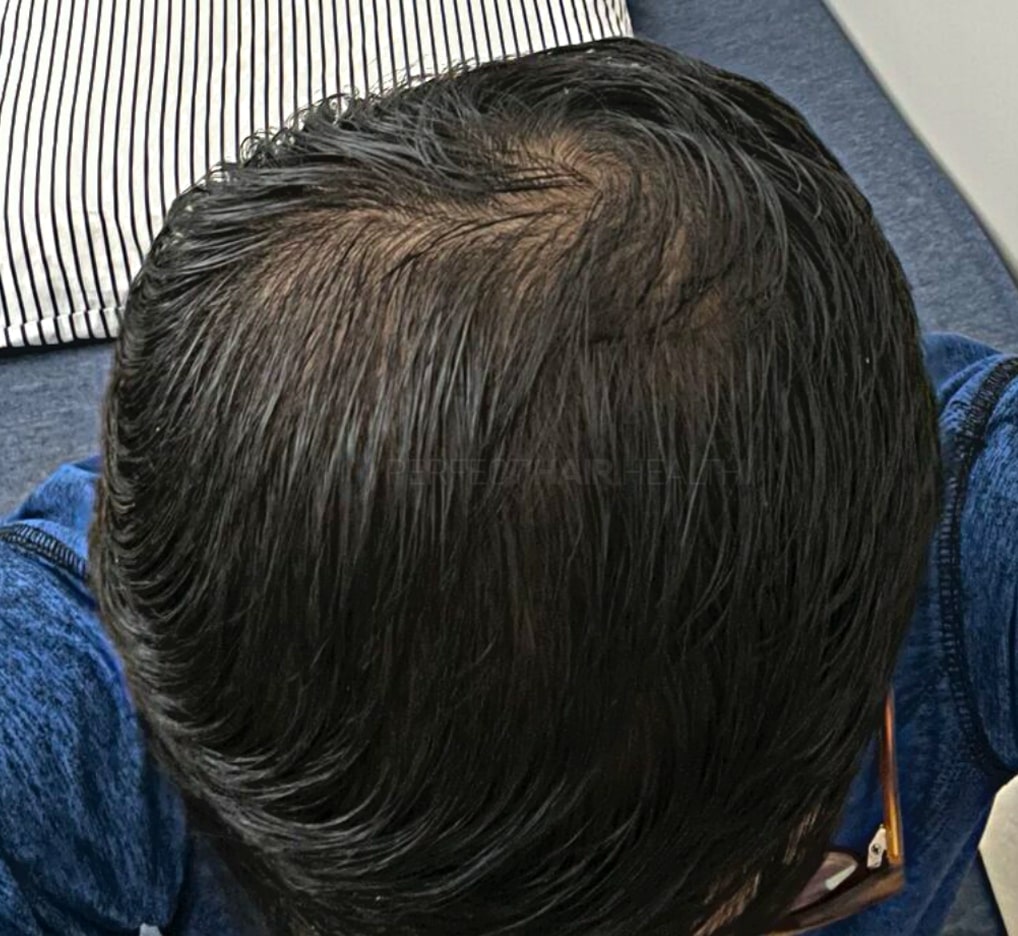 — Aayush, 20’s, Boston, MA
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
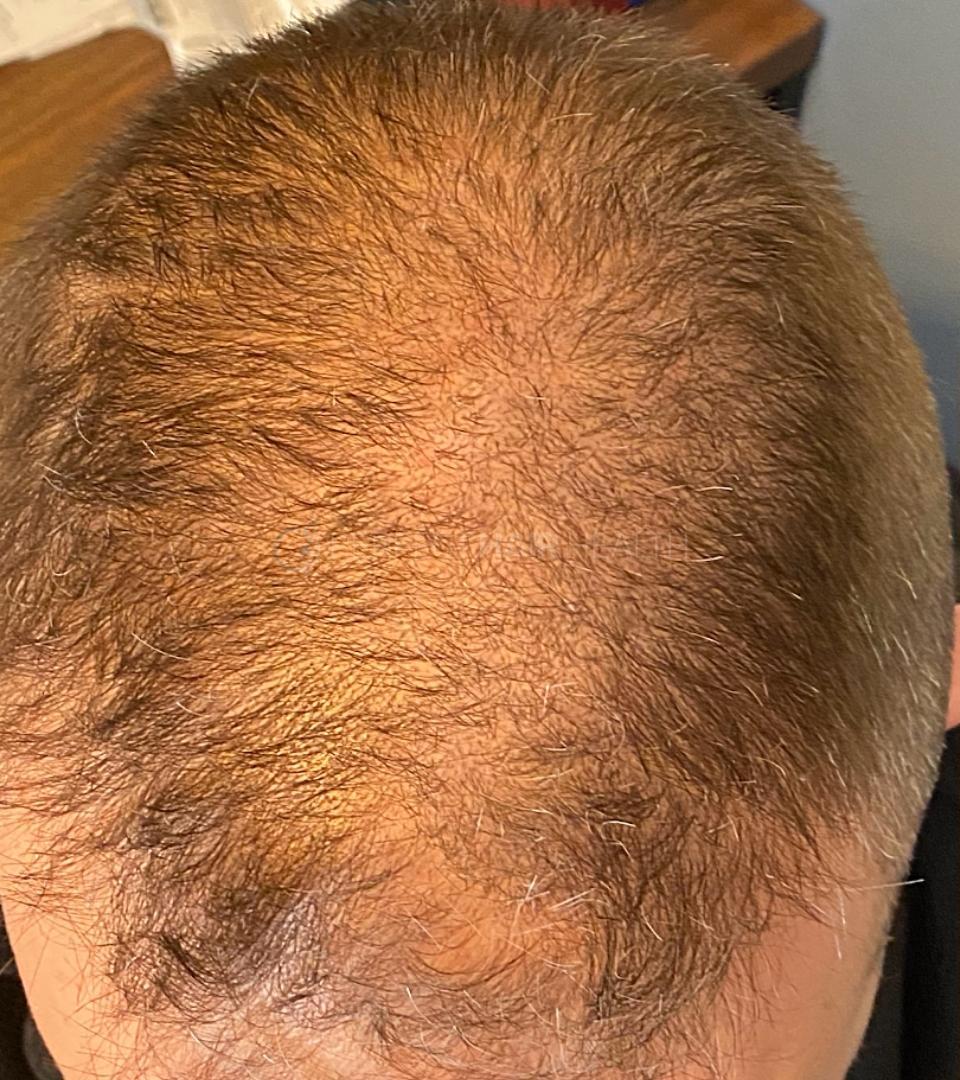
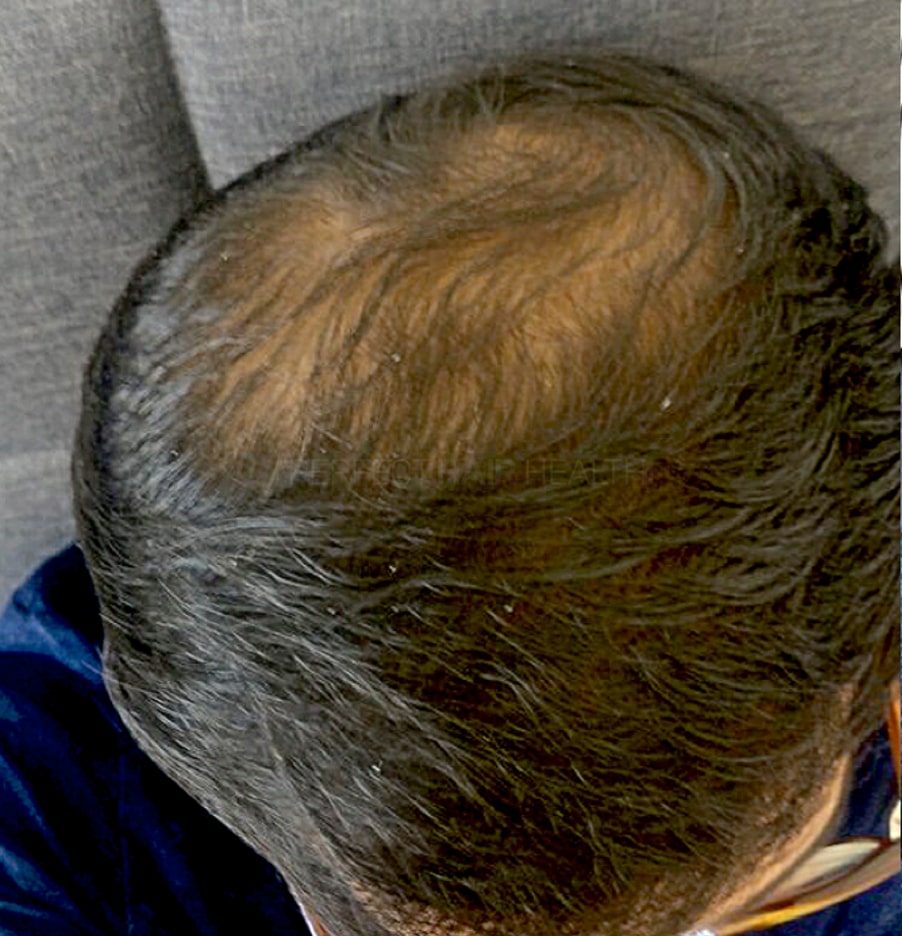
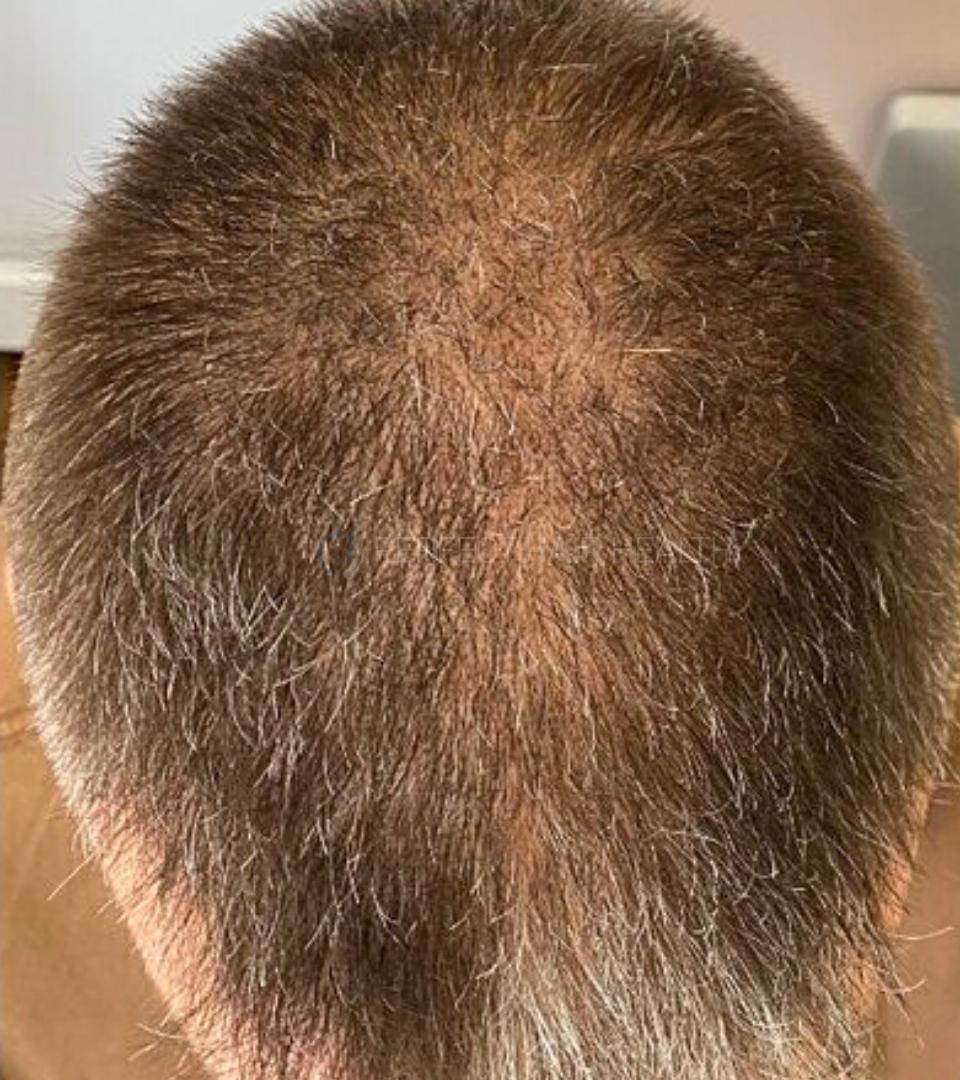 — Douglas, 50’s, Montréal, Canada
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement