- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
 Scroll DownPopular Treatments
Scroll DownPopular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Topical Finasteride Calculator
- Interactive Guide: What Causes Hair Loss?
- Free Guide: Standardized Scalp Massages
- 7-Day Hair Loss Email Course
- Ingredients Database
- Interactive Guide: Hair Loss Disorders
- Treatment Guides
- Product Lab Tests: Purity & Potency
- Evidence Quality Masterclass
More
Articles100+ free articles.
-
Cannabidiol (CBD) Increases Hair Counts By 246%? Not So Fast.
-
Creatine: Does It Worsen Hair Loss? It Depends On The Hair Loss Type.
-
Can Progesterone Improve Hair Regrowth?
-
CRABP2: Can This Gene Predict Regrowth From Retinoids?
-
BTD: Can This Gene Predict Regrowth From Biotin?
-
COL1A1: Can This Gene Predict Regrowth From Collagen Support?
-
2dDR For Hair Loss: What Do We Know So Far About This Sugar?
-
CYP19A1: Can This Gene Predict Regrowth From Hormone Therapy?
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
ArticlesRitlecitinib (Litfulo™) For Alopecia Areata: Benefits, Risks, & Hair Growth Outcomes
First Published Apr 24 2024Last Updated Oct 29 2024Pharmaceutical Researched & Written By:Sarah King, PhD
Researched & Written By:Sarah King, PhD Reviewed By:Rob English, Medical Editor
Reviewed By:Rob English, Medical Editor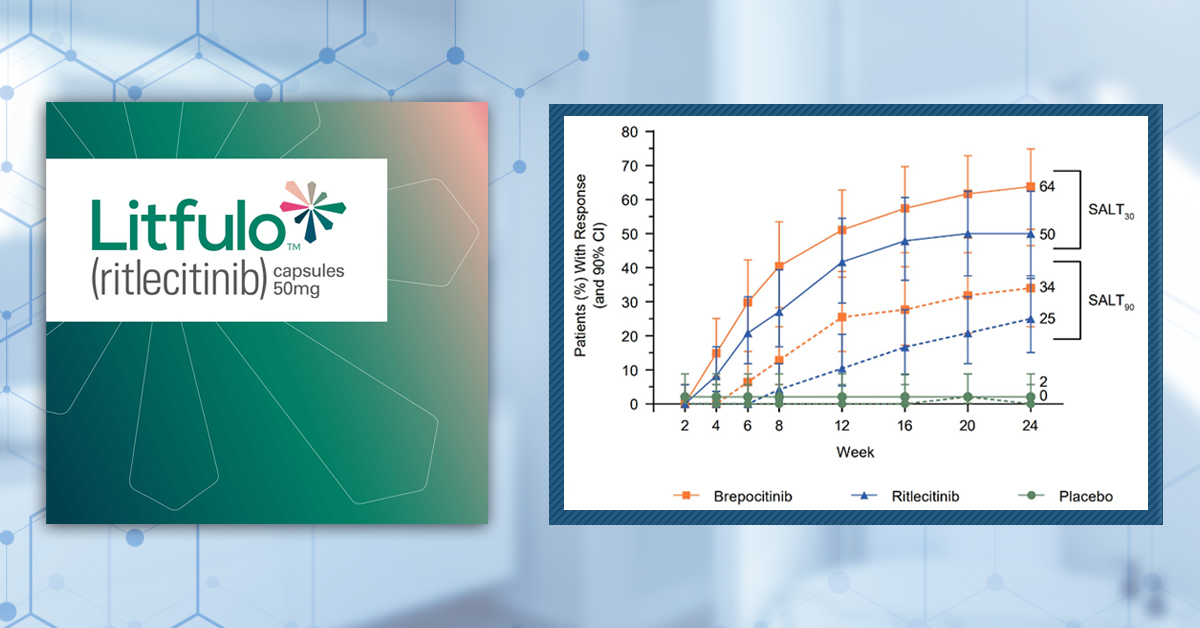
Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn MoreArticle Summary
In a recent milestone, a drug called ritlecitinib (brand name Litfulo™) was approved for treating alopecia areata (AA) in adolescents and adults. But is there high quality evidence supporting ritlecitinib? What are the long-term safety risks? And if it works, does this (relatively) new medication require lifelong use to prevent relapses of alopecia areata? In this article, we’ll discuss the science behind this hair loss drug.
Full Article
In a recent milestone, a drug called ritlecitinib (brand name Litfulo™) has been approved for treating alopecia areata (AA) in adolescents and adults. Ritlecitinib is a type of drug classified as a kinase inhibitor.[1]Pfizer, (2023), FDA Approves Pfizer’s LITFULO™ (Ritlecitinib) for Adults and Adolescents with Severe Alopecia Areata. Pfizer. Available at: … Continue reading In this article, we are going to explore how ritlecitinib works, its safety and efficacy, and its implications for treating hair loss.
Key Takeaways
- Drug. The small molecule ritlecitinib is an inhibitor of Janus-associated kinase 3 (JAK3) recently approved for treating alopecia areata (AA).
- Clinical Data. Clinical trials have demonstrated that ritlecitinib can effectively promote hair regrowth in patients with AA.
- In a phase 2a study conducted on 142 patients (98 women and 44 men), ritlecitinib treatments resulted in improved hair growth in a substantial number of patients – 25% of patients showed 90% or more regrowth by the end of the study and 50% of patients showing 30% or more regrowth by the end of this study.[2]King, B., Guttman-Yassky, E., Peeva, E., Banerjee, A., Sinclair, R., Pavel, A.B., Zhu, L., Cox, L.A., Craiglow, B., Chen, L., Banfield, C., Page, K., Zhang, W., Vincent, M.S. (2021). A phase 2a … Continue reading
- A phase 2b/3 study conducted on 718 patients (446 women and 272 men), further supported the efficacy of ritlecitinib, with patients who received the 30 mg or 50 mg doses responding to a greater extent than controls.[3]King, B., Zhang, X., Harcha, W.G., Szepietowski, J.C., Shapiro, J., Lynde, C., Mesinkovska, N.A., Zwillich, S.H., Napatalung, L., Wasjsbrot, D., Fayyad, R., Freyman, A., Mitra, D., Purohit, V., … Continue reading
- In a sub-study conducted within these clinical trials, ritlecitinib also reduced the expression of several biomarkers associated with alopecia areata, which correlated with the improvement in disease severity as assessed by SALT scores.[4]Guttman-Yasky, E., Pavel, A.B., Diaz, A., Zhang, N., Duca, E.D., Estrada, Y., King, B., Banarjee, A., Banfield, C., Cox, L.A., Dowty, M.E., Page, K., Vincent, M.S., Zhang, W., Zhu, L., Peeva, E. … Continue reading
- Safety. Ritlecitinib is FDA-approved for prescription use, so it should be safe if your doctor recommends it. However, some mild side effects are associated with its use, including upper respiratory tract infections, nasopharyngitis, and headaches. Serious side effects were rare in the clinical trials described above. However, there were instances of breast cancer and shingles during the clinical trials (2 out of 718 patients each).
- Evidence Quality: Ritlecitinib scored 76/100 for evidence quality by our metrics.
- Best Practices. Ritlectinib is specifically indicated for severe alopecia areata and is not suitable for other types of hair loss. According to the patient information leaflet, ritlecitinib should be given at dosages of 50 mg once daily. [5]LITFULO, (2023). Highlights of prescribing information. LITFULO. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215830s000lbl.pdf (Accessed: 18 September 2023)
What is Ritlecitinib?
Ritlecitinib is a small-molecule inhibitor of the enzyme janus-associated kinase (JAK) that was approved in the US in June 2023. [6]Ramirez-Marin & Tosti (2022), Evaluating the Therapeutic Potential of Ritlecitinib for the Treatment of Alopecia Areata. Drug Design, Development and Therapy. 16. 363-374. Available at: … Continue reading Over a year ago, another JAK inhibitor, baricitinib, was approved last year for severe AA. Now, there’s a second FDA-approved drug for this hair loss disorder, and of a similar class for its mechanisms of action.
The recommended dose of ritlecitinib is 50 mg orally once a day with or without food.[7]{Pfizer, (no date), LITFULO Medical Information. Pfizer. Available at: https://www.pfizermedicalinformation.com/en-us/litfulo/boxed-warning (Accessed: 14 September 2023)

Figure 1: Chemical structure of ritlecitinib.[8]Selleckchem (no date), Ritlecitinib. Selleckchem. Available at: https://www.selleckchem.com/products/pf-06651600.html (Accessed: 15 September 2023)
How Does Ritlecitinib Work?
Ritlecitinib comes under a class of drugs called Janus kinase (JAK) inhibitors. This is because it selectively inhibits a specific enzyme in the JAK family called JAK3. JAK3 is part of a cell signaling pathway called the JAK/STAT pathway, which transmits signals from outside the cell to the nucleus to regulate the expression of genes. This pathway is involved in the control of processes such as the maintenance of stem cells, the formation of new blood cells, and the inflammatory response.[9]Thomas, S.J., Snowden, J.A., Zeidler, M.P., and Danson, S.J. (2015), The role of JAK/STAT signaling in the pathogenesis, prognosis and treatment of solid tumors. British Journal of Cancer. 113. … Continue reading
The JAK/STAT pathway is activated by a range of growth factors and other signaling molecules, including a group of substances called cytokines. Cytokines are proteins that can modulate the immune system in different ways, depending onthe context.[10]Broussard, G., Damania, B. (2020), KSHV: Immune Modulation and Immunotherapy. Frontiers in Immunology. 10. 1-8. Available at: https://doi.org/10.3389/fimmu.2019.03084
In our article on baricitinib, we talk about the JAK/STAT pathway and how cytokine receptors can be described as transmembrane receptors – the receptors cross the cell membrane and have sections that can be found inside and outside the cell. The binding of the cytokine to the receptor on the outside of the cell causes a protein called JAK to bind to the inside portion of the receptor, leading it to undergo a process called trans-phosphorylation, in which a phosphate group is transferred from the receptor to the JAK protein(Figure 2).[11]Harrison, J.A. (2012), The JAK/STAT Pathway. Cold Spring Harbor Perspectives in Biology. 4(3). 1-3. Available at: https://doi.org/10.1101/cshperspect.a011205

Figure 2: Diagram showing the process of activation of STAT by JAK phosphorylation.[12]Luo, W., Li, Y.W., Jiang, L.J., Chen, Q., Wang, T., Ye, D.W. (2020). Targeting JAK-STAT signaling to Control Cytokine Release Syndrome in COVID-19. Trends in Pharmacological Sciences. 8. 531-543. … Continue reading
These JAK proteins, in turn, activate other pathways by phosphorylating proteins like the STATs (signal transducer and activator of protein transcription). When activated by JAK phosphorylation, STAT proteins undergo a change in shape, which allows them to move to the nucleus (called translocation), where they can then activate or deactivate specific genes involved in several cellular processes.[13]Awasthi, N., Liongue, C., Ward, A.C. (2021), STAT proteins: a kaleidoscope of canonical and non-canonical functions in immunity and cancer. Journal of Hematology & Oncology. 14(198). 1-17. … Continue reading
The response to JAK/STAT pathway activation can be different across different types of cells and tissue, depending on the specific type of proteins that make up the pathway and what other signaling pathways are operating in that cell. This provides a contextual element to JAK/STAT signaling.[14]Harrison, D.A., The JAK/STAT Pathway. Cold Spring Harbor Perspectives in Biology. 4(3). A011205. Available at: https://doi.org/10.1101/cshperspect.a011205
How does JAK/STAT signaling relate to AA?
The short answer: activation of the JAK/STAT pathway leads to hair loss caused by immune system activation.
How does AA develop? Let’s recap. Our hair follicles have something called ‘immune privilege’ – they are less subject to the immune response than most of the other areas of the body. This is thought to be in place to protect the stem-cell-containing area of the hair follicle, known as the bulge, which is required for new hair follicle growth in the hair cycle.[15]Azzawi, S., Penzi, L.R., Senna, M.M. (2018), Immune Privilege Collapse and Alopecia Development: Is Stress a Factor. Skin Appendage Disorders. 4(4).236-244. Available at: … Continue reading In AA, something triggers a breakdown of the immune privilege of the hair follicle. The exact cause of this breakdown is not fully understood, but it may involve genetic factors, environmental triggers, or a combination of both.
When the immune privilege is compromised, a group of immune cells called T cells begin to attack the hair follicle. As mentioned earlier, the JAK/STAT pathway plays a critical role in transmitting signals within cells. When the immune system attacks hair follicles, the JAK/STAT pathway is activated, sending signals instructing cells to participate in the attack. As a result, the cells around the hair follicles respond to these signals by causing inflammation and damaging the hair follicle. This disrupts the normal hair growth cycle and leads to hair loss in the affected areas (Figure 3).[16]Kumar, N., Kuang, L., Villa, R., Kumar, P., Mishra, J. (2021), Mucosal Epithelial Jak Kinases in Health and Diseases. Mediators of Inflammation. 1-17. https://doi.org/10.1155/2021/6618924

Figure 3: The collapse of immune privilege in the anagen hair follicle during AA.[17]Lensin, M., Jabbari, A. (2022), An Overview of JAK/STAT Pathways and JAK Inhibition in Alopecia Areata. Frontiers in Immunology. 13. 1-17. Available at: https://doi.org/10.3389/fimmu.2022.955035
Why target JAK3 specifically?
JAK3 is particularly important in the development and function of T cells.[18]Sohn, S.J., Forbush, K.A., Nguyen, N., Witthuhn, B., Nosaka, T., Ihie, J.N., Perimutter, R.M. (1998). Requirement for JAK3 in mature T cells: Its role in regulation of T cell homeostasis. The Journal … Continue reading By targeting JAK3, ritlecitinib may help inhibit T cell activity without affecting other immune cells as strongly. This targeted approach may help to reduce the autoimmune response while preserving some aspects of the immune system’s ability to fight infections.[19]Dai, Z., Sezin, T., Chang, Y., Lee, E.Y., Wang, E.H.C., Christiano, A.M. (2022), Induction of T cell exhaustion by JAK1/3 inhibition in the treatment of alopecia areata. Frontiers in Immunology. 13. … Continue reading
Furthermore, Targeting JAK3 may provide a more precise therapeutic approach for AA. Since JAK3 is primarily involved in immune responses mediated by certain subsets of T cells, it could potentially lead to fewer off-target effects on other immune system functions. Of course, this precision is desirable to avoid compromising the immune system’s ability to protect against infections. Inhibiting JAK1/2 may have broader effects on the immune system, potentially leading to more significant side effects. For example, JAK1/2 inhibitors might interfere with the body’s response to infections or impact other physiological processes.[20]Sardana, K., Bathula, S., Khurana, A. (2023), Which is the Ideal JAK Inhibitor for Alopecia Areata – Baricitinib, Tofacitinib, Ritlecitinib, or Ifidancitinib – Revisiting the … Continue reading By specifically targeting JAK3, the goal is to minimize unwanted side effects while still addressing the autoimmune component of the AA disease mechanism.
Is Ritlecitinib Effective at Treating Alopecia Areata?
Several clinical trials have either already been carried out using ritlecitinib or are ongoing. These trials have been conducted in adults, adolescents, and children, either with AA or cicatricial alopecia (which is similar to AA in terms of disease mechanism, although there are important differences). In total, we could find 2 completed clinical trials with a total number of patients included being 789. We will also cover one of the studies that were conducted at the same time as these trials with the same patients.
The first study was a phase 2a randomized, placebo-controlled study to evaluate the efficacy and safety of ritlecitinib and a similar drug, brepocitinib, in AA.[21]King, B., Guttman-Yassky, E., Peeva, E., Banerjee, A., Sinclair, R., Pavel, A.B., Zhu, L., Cox, L.A., Craiglow, B., Chen, L., Banfield, C., Page, K., Zhang, W., Vincent, M.S. (2021). A phase 2a … Continue reading 142 participants took part (98 female and 44 male) with an average age of 36. Participants were treated with 200 mg of ritlecitinib or 60 mg of baricitinib once daily for four weeks. Then, they were treated with a maintenance dose of 50 mg (ritlecitinib) or 30 mg (baricitinib) for the next 20 weeks. The results were promising for patients with AA who started the study with over 50% of scalp hair loss. Both medications resulted in significant hair regrowth, with 25% (ritlecitinib group) and 34% (baricitinib group) of patients achieving near-complete hair regrowth as measured by Severity of Alopecia Tool (SALT90) scores compared to the placebo group (Figure 4).
The SALT tool is validated for measuring the extent of hair loss in AA with 0% meaning no hair loss, and 100% meaning complete hair loss (with several grades in between).[22]Wyrwich, K.W., Kitchen, H., Knight, S., Aldhouse, N.V.J., Macey, J., Nunes, F.P., Dutronc, Y., Mesinkovska, N., Ko, J.M., King, B.A. (2020). The Alopecia Areata Investigator Global Assessment Scale: … Continue reading The SALT90 score, however, measures the percentage of people who achieved at least 90% of hair regrowth.

Figure 4: Effect of Brepocitinib, Ritlectinib, or a placebo on SALT scores in patients with alopecia areata over 24 weeks. SALT30 = % of patients who showed a SALT score over 30%. SALT90 = % of patients who showed a SALT score over 90%.[23]King, B., Guttman-Yassky, E., Peeva, E., Banerjee, A., Sinclair, R., Pavel, A.B., Zhu, L., Cox, L.A., Craiglow, B., Chen, L., Banfield, C., Page, K., Zhang, W., Vincent, M.S. (2021). A phase 2a … Continue reading
Adverse events were reported in all three treatment groups, with common events including upper respiratory tract infection, nasopharyngitis, headache, acne, and nausea. Serious adverse events were only noted in the brepocitinib group, which could indicate that it may induce more hair regrowth than ritlecitinib but may not be as safe to use.
So, to summarize, although brepocitinib performed better, ritlecitinib also improved hair regrowth scores in patients with AA and appeared to be better tolerated than brepocitinib.
This particular clinical trial also contained a further sub-study to further evaluate the efficacy and safety of ritlecitinib and brepocitinib.[24]Guttman-Yasky, E., Pavel, A.B., Diaz, A., Zhang, N., Duca, E.D., Estrada, Y., King, B., Banarjee, A., Banfield, C., Cox, L.A., Dowty, M.E., Page, K., Vincent, M.S., Zhang, W., Zhu, L., Peeva, E. … Continue reading The study involved taking scalp biopsy samples from patients with AA at baseline and analyzing changes in specific biomarkers between baseline and weeks 12 and 24 of treatment.
Ritlecitinib significantly shifted gene expression in the scalp towards an improved, non-lesional profile. This improvement exceeded 100% of baseline at week 24, indicating a strong positive response to the treatment. Furthermore, the treatment led to a downregulation of inflammatory markers associated with T cells, T cell activation, and other immune response markers. These changes also correlated with an improvement in the SALT score (Figure 5).

Figure 5: Overall percentage improvement in the lesional scalp for each treatment group at weeks 12 and 24. P=<0.001 at weeks 12 and 24 comparisons with placebo and comparisons between treatment groups.[25]Guttman-Yasky, E., Pavel, A.B., Diaz, A., Zhang, N., Duca, E.D., Estrada, Y., King, B., Banarjee, A., Banfield, C., Cox, L.A., Dowty, M.E., Page, K., Vincent, M.S., Zhang, W., Zhu, L., Peeva, E. … Continue reading
However, the most recent study (just prior to ritlecitinib becoming FDA approved) was a 48-week phase 2b/3 randomized, double-blind, placebo-controlled clinical trial.[26]King, B., Zhang, X., Harcha, W.G>, Szepietowski, J.C., Shapiro, J., Lynde, C., Mesinkovska, N.A., Zwillich, S.H., Napatalung, L., Wajsbrot, D., Fayyad, R., Freyman, A., Mitra, D., Purohit, V., … Continue reading The trial was conducted across118 sites in 18 countries, with 718 patients (446 women, 272 men) aged 12 years and older with alopecia areata and at least 50% scalp hair loss – as measured by SALT severity scores.
The patients were assigned to either an oral ritlecitinib or placebo treatment, given once daily for 24 weeks. Several doses of ritlecitinib were tested, along with a variable 4-week loading dose and were followed by a 24-week extension period, during which the ritlecitinib group continued their treatment, and the placebo-treated group switched to ritlecitinib (Figure 6).

Figure 6: The clinical trial study design.[27]King, B., Zhang, X., Harcha, W.G., Szepietowski, J.C., Shapiro, J., Lynde, C., Mesinkovska, N.A., Zwillich, S.H., Napatalung, L., Wasjsbrot, D., Fayyad, R., Freyman, A., Mitra, D., Purohit, V., … Continue reading
The researchers found that patients on the 30 mg or 50 mg doses of ritlecitinib exhibited the highest regrowth scores, and including a loading dose resulted in further improvement. Response rates based on a SALT score of 20% or less at week 24 were significantly higher in ritlecitinib groups compared to the placebo (Figure 7A):
- 29.1% difference for 200 mg + 50 mg
- 20.8% difference for 200 mg + 30 mg
- 21.9% difference for 50 mg
- 12.8% difference for 30 mg
The response rates, based on a SALT score of 10 or less at week 24, were also significantly higher in ritlecitinib groups compared to the placebo (Figure 7B). Furthermore, the proportion of patients with a Patient Global Impression of Change (PGI-C) response of moderately or greatly improved was greater with ritlecitinib treatment compared to placebo at week 24 (Figure 7C). The PGI-C reflects a patient’s belief about the efficacy of a treatment.[28]Ferguson, L., Scheman, J. (2009). Patient global impression of change scores within the chronic pain rehabilitation program. The Journal of Pain. 10(4). S73. Available at: … Continue reading Regrowth of eyelashes and eyebrows was also observed with ritlecitinib treatment (Figures 7D/7E), with responses continuing up to week 48 in all ritlecitinib groups.

Figure 7: Efficacy of ritlecitinib treatment with or without loading doses and compared to placebo groups. (A): Response based on a SALT score of 20 or less. (B) Response based on a SALT score of 10 or less. (C): PGI-C response. (D): Eyebrow response. (E): Eyelash response. (F): Representative photo of a patient at baseline and week 24 with significant growth observed.[29]King, B., Zhang, X., Harcha, W.G., Szepietowski, J.C., Shapiro, J., Lynde, C., Mesinkovska, N.A., Zwillich, S.H., Napatalung, L., Wasjsbrot, D., Fayyad, R., Freyman, A., Mitra, D., Purohit, V., … Continue reading
So, to summarize, ritlecitinib was effective in promoting hair regrowth and was generally well tolerated (more on that below) in patients with alopecia areata. There is a longer-term efficacy study currently ongoing called ALLEGRO-LT, which aims to evaluate the safety and efficacy of ritlecitinib over a period of 60 months (5 years).[30]Clinical Trials (2023) Long-term PF-06651600 for the Treatment of Alopecia Areata (ALLEGRO-LT). NIH. Available at: … Continue reading This trial is due to finish in 2026 and will hopefully provide the long-term efficacy and safety data that is needed.
Is Ritlecitinib Safe?
The overall safety profile was observed throughout the study, and it was determined that ritlecitinib was generally well-tolerated across all doses and showed a favorable safety profile. The most common adverse effects (occurring in at least 10% of patients in any treatment group) included:
- Upper respiratory tract infection – 8.01% of all patients who took ritlecitinib.
- Nasopharyngitis – 13.01% of all patients who took ritlecitinib.
- Headache – 10.96% of all patients who took ritlecitinib
A total of 16 more serious adverse events were reported in 14 patients. These included:
- Infections (Five serious infections were reported. Notably, one patient experienced empyema (fluid between the lungs) and sepsis, while two cases of shingles occurred.
- Malignancies: Two cases of breast cancer were reported, one of which was deemed related to the treatment by an investigator.
- Other serious events were appendicitis, diverticulitis, and pulmonary embolism.
Treatment with ritlecitinib also led to changes in hematological parameters, including:
- Decrease in hemoglobin levels.
- Transient decrease in platelet count.
- Early decrease in absolute lymphocyte levels, particularly in groups with the 200 mg loading dose.
- Early decreases in natural killer cell counts, most apparent in groups with a 200 mg loading dose.
So 14/718 patients experienced a more serious adverse effect – so roughly 2% of patients, or 1 in 50. While you may consider these ‘good odds’, it is important to consider these potential adverse effects when making a treatment decision and always consult a physician.
Is Ritlecitinib for Me?
Ritlecitinib is an exciting new treatment; however, it is important to remember that it is for the treatment of severe alopecia areata only and will not work for other hair loss types like androgenetic alopecia, as the mechanisms of hair loss are very different. You may want to try this treatment if:
- You have severe alopecia areata.
- You have tried other treatments with no success.
- You have spoken to your physician, and they have suggested it based on your alopecia severity.
References[+]
References ↑1 Pfizer, (2023), FDA Approves Pfizer’s LITFULO™ (Ritlecitinib) for Adults and Adolescents with Severe Alopecia Areata. Pfizer. Available at: https://www.pfizer.com/news/press-release/press-release-detail/fda-approves-pfizers-litfulotm-ritlecitinib-adults-and (Accessed: 14 September 2023) ↑2, ↑23 King, B., Guttman-Yassky, E., Peeva, E., Banerjee, A., Sinclair, R., Pavel, A.B., Zhu, L., Cox, L.A., Craiglow, B., Chen, L., Banfield, C., Page, K., Zhang, W., Vincent, M.S. (2021). A phase 2a randomized, placebo-controlled study to evaluate the efficacy and safety of the oral janus kinase inhibitors ritlecitinib and brepocitinib in alopecia areata: 24-week results. Journal of the American Academy of Dermatology. 85(2). 379-387. Available at: https://doi.10.1016/j.jaad.2021.03.050 ↑3 King, B., Zhang, X., Harcha, W.G., Szepietowski, J.C., Shapiro, J., Lynde, C., Mesinkovska, N.A., Zwillich, S.H., Napatalung, L., Wasjsbrot, D., Fayyad, R., Freyman, A., Mitra, D., Purohit, V., Sinclair, R., Wolk, R. (2023). Efficacy and Safety of Ritlecitinib in adults and adolescents with alopecia areata: a randomized, double-blind, multicentre, phase 2b-3 trial. The Lancet. 401. 1518-1529. Available at: https://doi.org/10.1016/S0140-6736(23)00222-2 ↑4, ↑24, ↑25 Guttman-Yasky, E., Pavel, A.B., Diaz, A., Zhang, N., Duca, E.D., Estrada, Y., King, B., Banarjee, A., Banfield, C., Cox, L.A., Dowty, M.E., Page, K., Vincent, M.S., Zhang, W., Zhu, L., Peeva, E. (2022), Ritlecitinib and brepocitinib demonstrate significant improvement in scalp alopecia areata biomarkers. Atopic Dermatitis and Inflammatory Skin Disease. 149(4). P1318-1328. Available at: https://doi.org/10.1016/j.jaci.2021.10.036 ↑5 LITFULO, (2023). Highlights of prescribing information. LITFULO. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215830s000lbl.pdf (Accessed: 18 September 2023) ↑6 Ramirez-Marin & Tosti (2022), Evaluating the Therapeutic Potential of Ritlecitinib for the Treatment of Alopecia Areata. Drug Design, Development and Therapy. 16. 363-374. Available at: https://doi.org/10.2147/DDDT.S334727 ↑7 {Pfizer, (no date), LITFULO Medical Information. Pfizer. Available at: https://www.pfizermedicalinformation.com/en-us/litfulo/boxed-warning (Accessed: 14 September 2023) ↑8 Selleckchem (no date), Ritlecitinib. Selleckchem. Available at: https://www.selleckchem.com/products/pf-06651600.html (Accessed: 15 September 2023) ↑9 Thomas, S.J., Snowden, J.A., Zeidler, M.P., and Danson, S.J. (2015), The role of JAK/STAT signaling in the pathogenesis, prognosis and treatment of solid tumors. British Journal of Cancer. 113. 365-371. Available at: https://doi.org/10.1038/bjc.2015.233 ↑10 Broussard, G., Damania, B. (2020), KSHV: Immune Modulation and Immunotherapy. Frontiers in Immunology. 10. 1-8. Available at: https://doi.org/10.3389/fimmu.2019.03084 ↑11 Harrison, J.A. (2012), The JAK/STAT Pathway. Cold Spring Harbor Perspectives in Biology. 4(3). 1-3. Available at: https://doi.org/10.1101/cshperspect.a011205 ↑12 Luo, W., Li, Y.W., Jiang, L.J., Chen, Q., Wang, T., Ye, D.W. (2020). Targeting JAK-STAT signaling to Control Cytokine Release Syndrome in COVID-19. Trends in Pharmacological Sciences. 8. 531-543. Available at: https://doi.org/10.1016/kj.tips.2020.06.007 ↑13 Awasthi, N., Liongue, C., Ward, A.C. (2021), STAT proteins: a kaleidoscope of canonical and non-canonical functions in immunity and cancer. Journal of Hematology & Oncology. 14(198). 1-17. https://doi.org/10.1186/s13045-021-01214-y ↑14 Harrison, D.A., The JAK/STAT Pathway. Cold Spring Harbor Perspectives in Biology. 4(3). A011205. Available at: https://doi.org/10.1101/cshperspect.a011205 ↑15 Azzawi, S., Penzi, L.R., Senna, M.M. (2018), Immune Privilege Collapse and Alopecia Development: Is Stress a Factor. Skin Appendage Disorders. 4(4).236-244. Available at: https://doi.org/10.1159/0000485080 ↑16 Kumar, N., Kuang, L., Villa, R., Kumar, P., Mishra, J. (2021), Mucosal Epithelial Jak Kinases in Health and Diseases. Mediators of Inflammation. 1-17. https://doi.org/10.1155/2021/6618924 ↑17 Lensin, M., Jabbari, A. (2022), An Overview of JAK/STAT Pathways and JAK Inhibition in Alopecia Areata. Frontiers in Immunology. 13. 1-17. Available at: https://doi.org/10.3389/fimmu.2022.955035 ↑18 Sohn, S.J., Forbush, K.A., Nguyen, N., Witthuhn, B., Nosaka, T., Ihie, J.N., Perimutter, R.M. (1998). Requirement for JAK3 in mature T cells: Its role in regulation of T cell homeostasis. The Journal of Immunology. 160(5). 2130-2138. Available at: https://doi.org/10.4049/jimmunol.160.5.2130 ↑19 Dai, Z., Sezin, T., Chang, Y., Lee, E.Y., Wang, E.H.C., Christiano, A.M. (2022), Induction of T cell exhaustion by JAK1/3 inhibition in the treatment of alopecia areata. Frontiers in Immunology. 13. 1-14. Available at: https://doi.org/10.3389/fimmu.2022.955038 ↑20 Sardana, K., Bathula, S., Khurana, A. (2023), Which is the Ideal JAK Inhibitor for Alopecia Areata – Baricitinib, Tofacitinib, Ritlecitinib, or Ifidancitinib – Revisiting the Immunomechanisms of the JAK Pathway. Indian Dermatology Online Journal. 14. 465-474. Available at: https://doi.org/10.4103/idoj.idoj_452_22 ↑21 King, B., Guttman-Yassky, E., Peeva, E., Banerjee, A., Sinclair, R., Pavel, A.B., Zhu, L., Cox, L.A., Craiglow, B., Chen, L., Banfield, C., Page, K., Zhang, W., Vincent, M.S. (2021). A phase 2a randomized, placebo-controlled study to evaluate the efficacy and safety of the oral janus kinase inhibitors ritlecitinib and brepocitinib in alopecia areata: 24-week results. Journal of the American Academy of Dermatology. 85(2). 379-387. Available at: https://doi.org/10.1016/j.jaad.2021.03.050 ↑22 Wyrwich, K.W., Kitchen, H., Knight, S., Aldhouse, N.V.J., Macey, J., Nunes, F.P., Dutronc, Y., Mesinkovska, N., Ko, J.M., King, B.A. (2020). The Alopecia Areata Investigator Global Assessment Scale: A Measure for Evaluating Clinically Meaningful Success in Clinical Trials. 183(4). 702-709. Available at: https://doi.org/10.1111/bjd.18883 ↑26 King, B., Zhang, X., Harcha, W.G>, Szepietowski, J.C., Shapiro, J., Lynde, C., Mesinkovska, N.A., Zwillich, S.H., Napatalung, L., Wajsbrot, D., Fayyad, R., Freyman, A., Mitra, D., Purohit, V., Sinclair, R., Wolk, R. (2023). Efficacy and safety of ritlecitinib in adults and adolescents with alopecia areata: a randomized, double-blind, multicentre, phase 2b-3 trial. The Lancet. 401(10387). 1518-1529. Available at: https://doi.org/10.1016/S0140-6736(23)002222-2 ↑27, ↑29 King, B., Zhang, X., Harcha, W.G., Szepietowski, J.C., Shapiro, J., Lynde, C., Mesinkovska, N.A., Zwillich, S.H., Napatalung, L., Wasjsbrot, D., Fayyad, R., Freyman, A., Mitra, D., Purohit, V., Sinclair, R., Wolk, R. (2023). Efficacy and Safety of Ritlecitinib in Adults and Adolescents with Alopecia Areata: a Randomized, Double-Blind, Multicentre, Phase 2b-3 Trial. The Lancet. 401. 1518-1529. Available at: https://doi.org/10.1016/S0140-6736(23)00222-2 ↑28 Ferguson, L., Scheman, J. (2009). Patient global impression of change scores within the chronic pain rehabilitation program. The Journal of Pain. 10(4). S73. Available at: https://doi.org/10.1016/j.pain.2009.01.258 ↑30 Clinical Trials (2023) Long-term PF-06651600 for the Treatment of Alopecia Areata (ALLEGRO-LT). NIH. Available at: https://clinicaltrials.gov/study/NCT04006457?term=ALLEGRO-LT&checkSpell=false&rank=1 (Accessed: 18 September 2023) Want help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Learn More
Sarah King, PhD
Dr. Sarah King is a researcher & writer who holds a BSc in Medical Biology, an MSc in Forensic Biology, and a Ph.D. in Molecular and Cellular Biology. While at university, Dr. King’s research focused on cellular aging and senescence through NAD-dependent signaling – along with research into prostaglandins and their role in hair loss. She is a co-author on several upcoming manuscripts with the Perfect Hair Health team.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
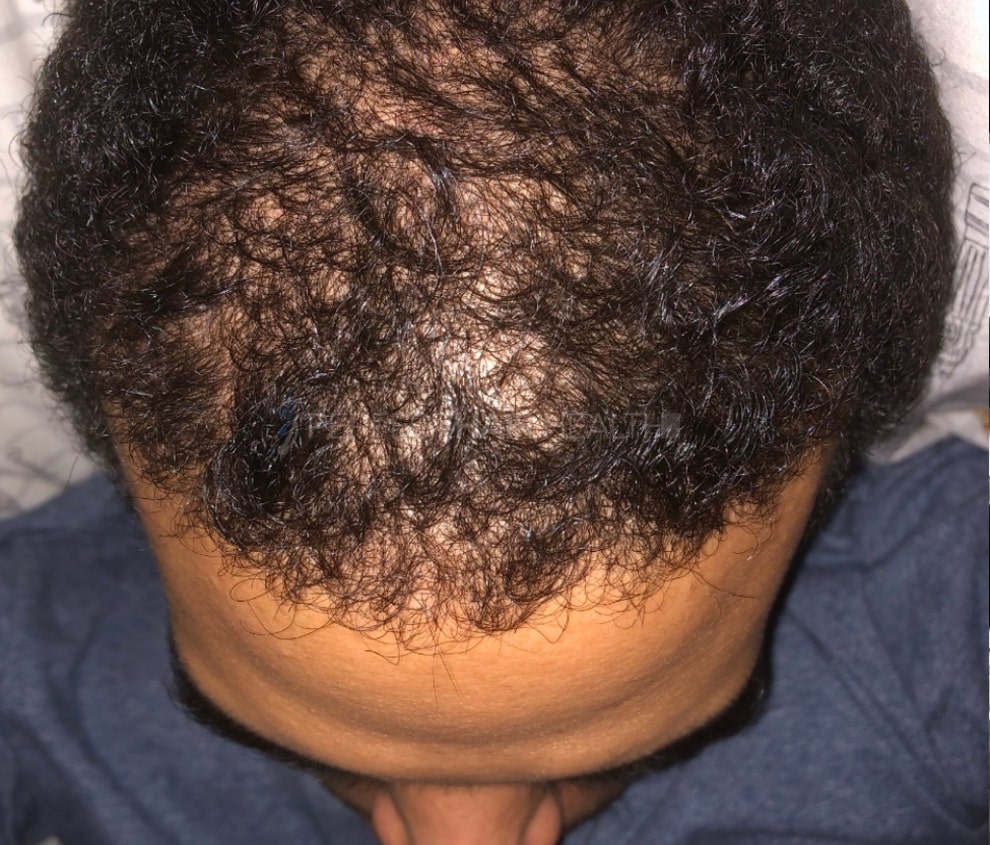
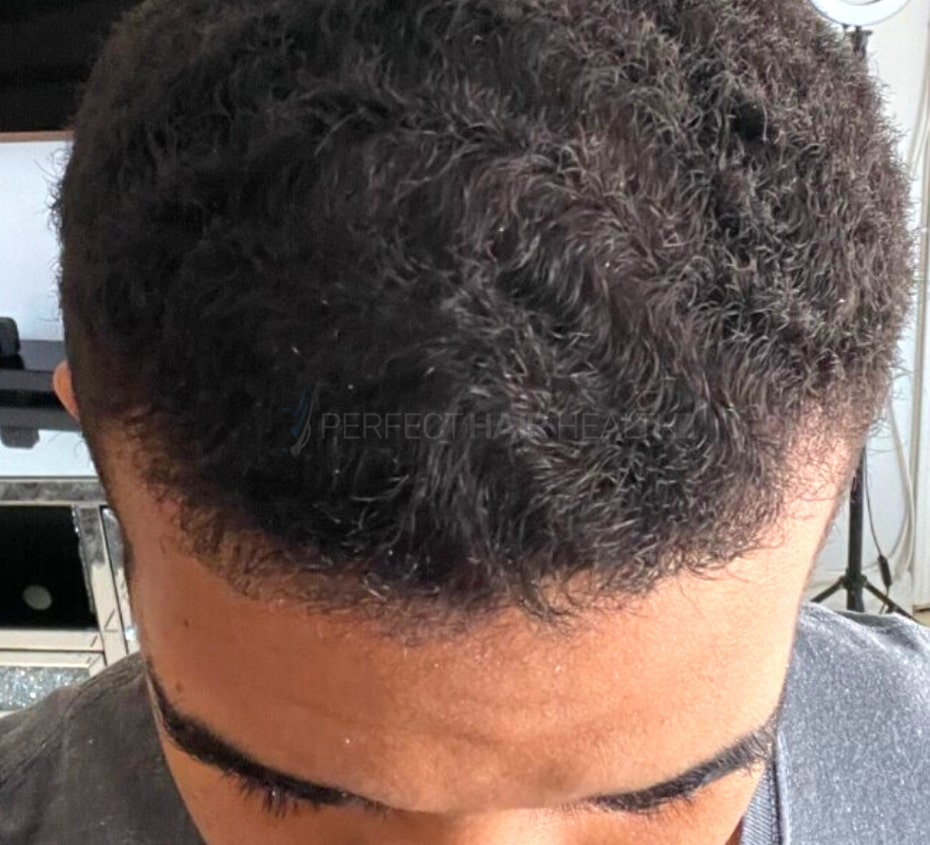 — RDB, 35, New York, U.S.A.
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
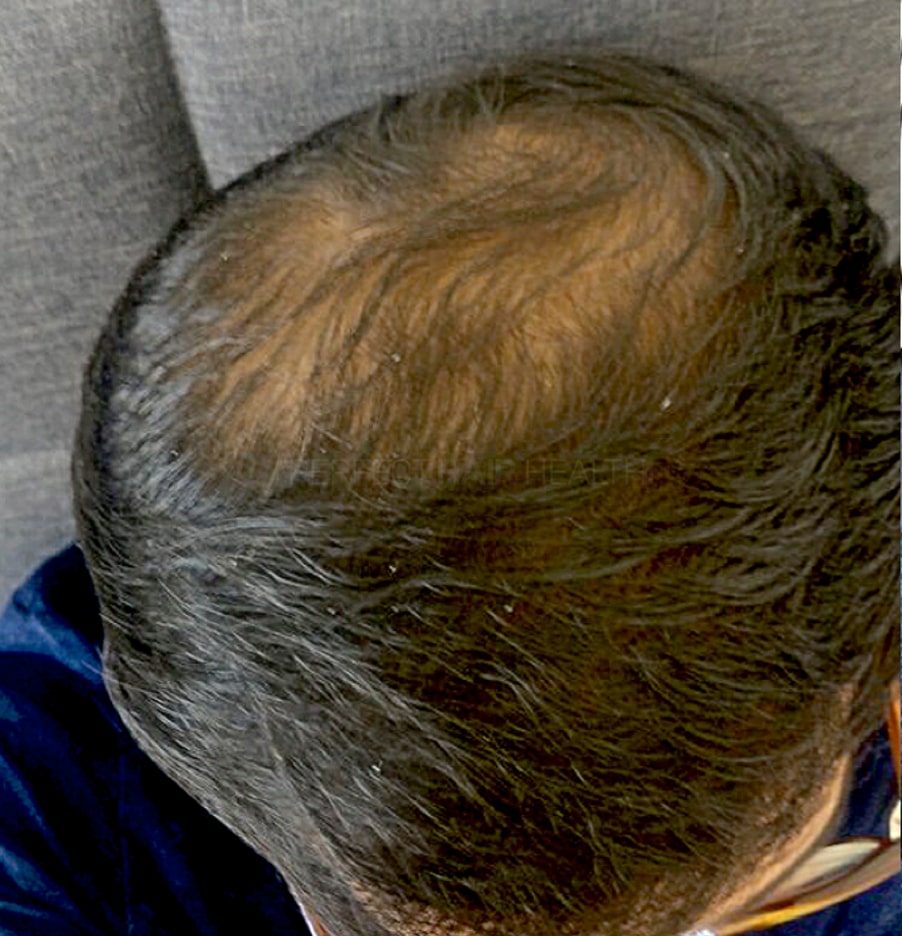
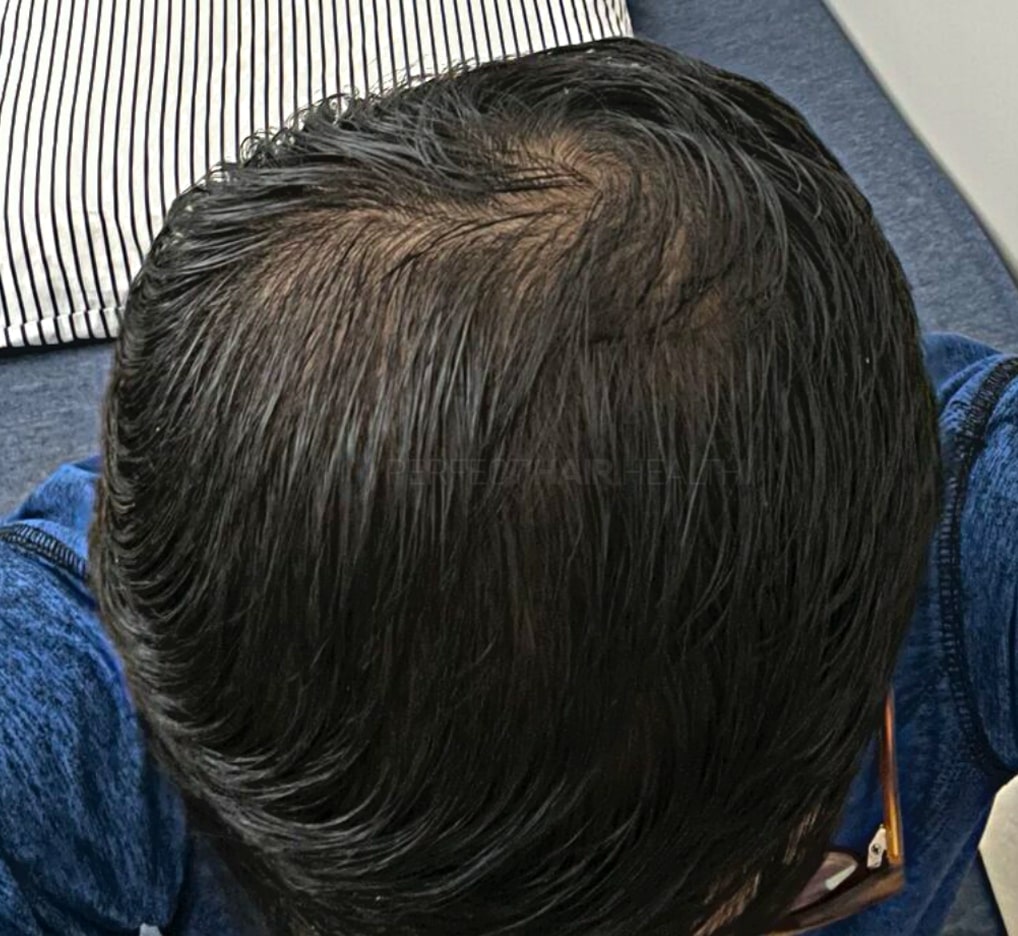 — Aayush, 20’s, Boston, MA
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
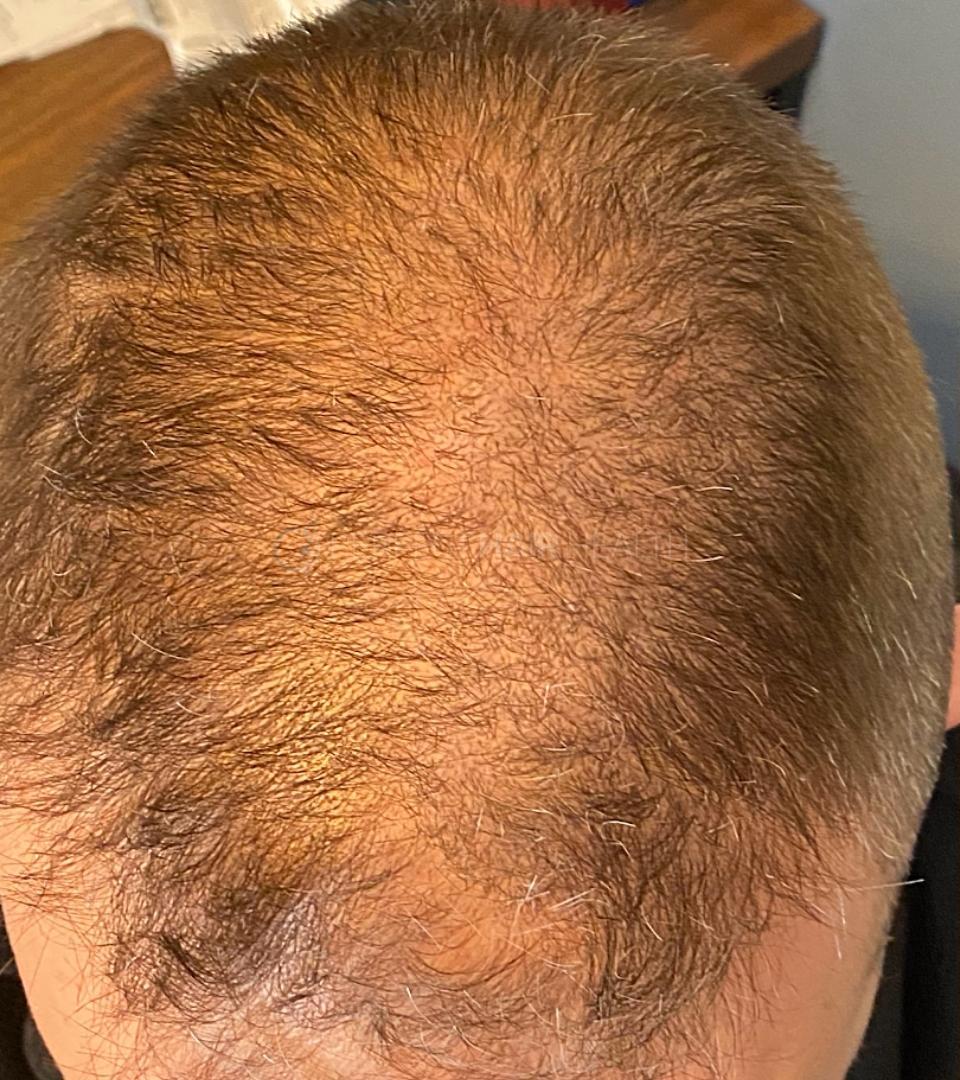
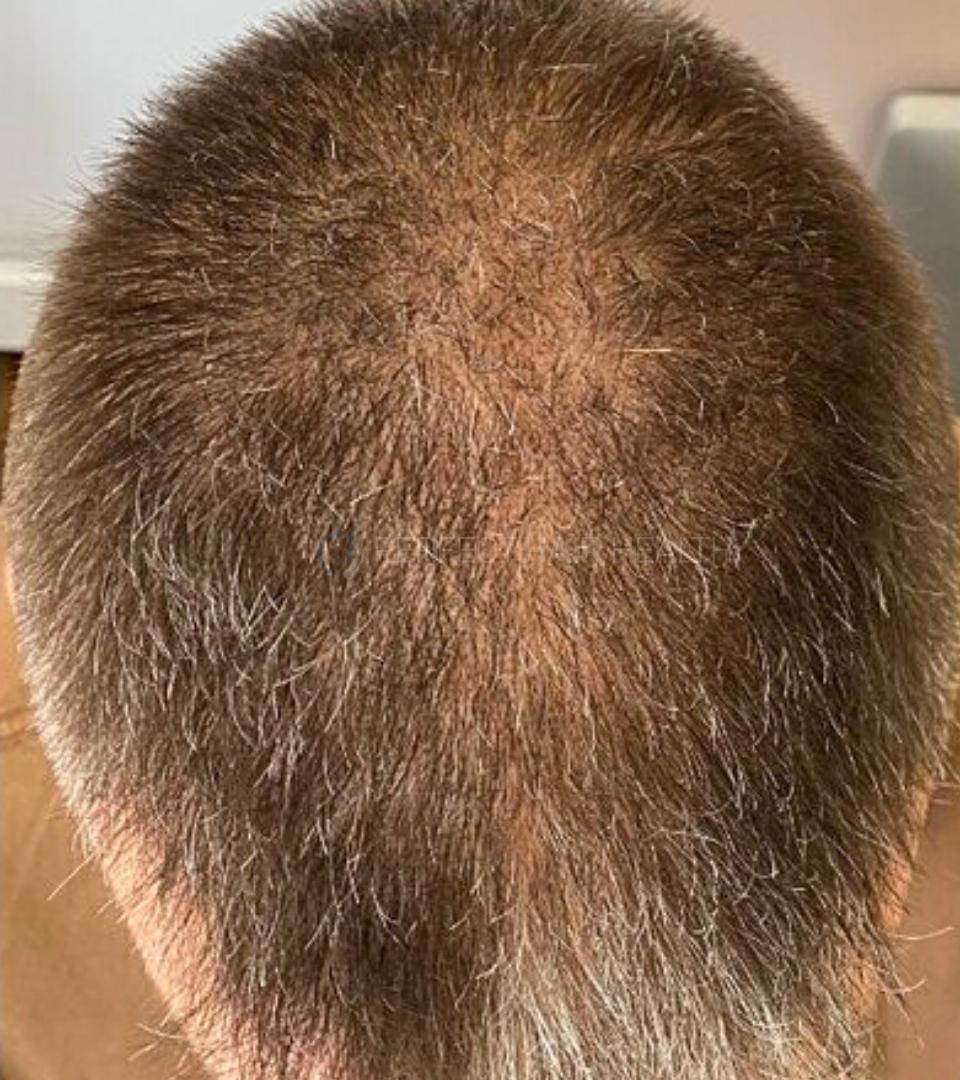 — Douglas, 50’s, Montréal, Canada
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement