- About
- Mission Statement
Education. Evidence. Regrowth.
- Education.
Prioritize knowledge. Make better choices.
- Evidence.
Sort good studies from the bad.
- Regrowth.
Get bigger hair gains.
Team MembersPhD's, resarchers, & consumer advocates.
- Rob English
Founder, researcher, & consumer advocate
- Research Team
Our team of PhD’s, researchers, & more
Editorial PolicyDiscover how we conduct our research.
ContactHave questions? Contact us.
Before-Afters- Transformation Photos
Our library of before-after photos.
- — Jenna, 31, U.S.A.
I have attached my before and afters of my progress since joining this group...
- — Tom, 30, U.K.
I’m convinced I’ve recovered to probably the hairline I had 3 years ago. Super stoked…
- — Rabih, 30’s, U.S.A.
My friends actually told me, “Your hairline improved. Your hair looks thicker...
- — RDB, 35, New York, U.S.A.
I also feel my hair has a different texture to it now…
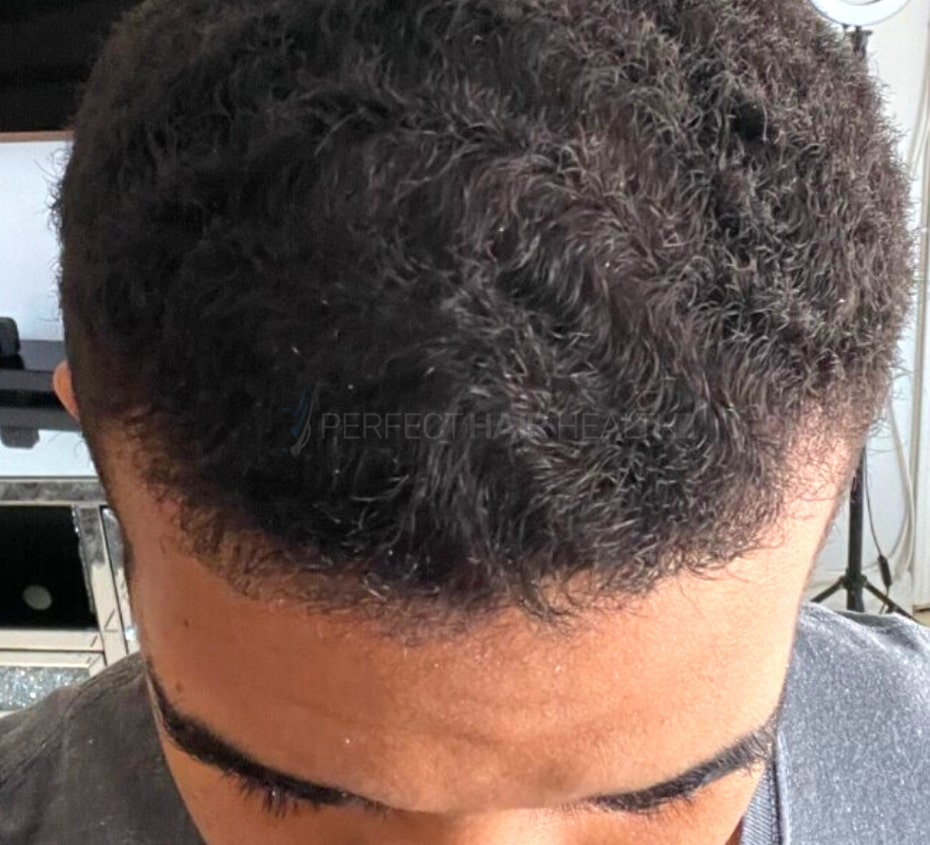
- — Aayush, 20’s, Boston, MA
Firstly thank you for your work in this field. I am immensely grateful that...
- — Ben M., U.S.A
I just wanted to thank you for all your research, for introducing me to this method...
- — Raul, 50, Spain
To be honest I am having fun with all this and I still don’t know how much...
- — Lisa, 52, U.S.
I see a massive amount of regrowth that is all less than about 8 cm long...
Client Testimonials150+ member experiences.
 Scroll DownPopular Treatments
Scroll DownPopular Treatments- Treatments
Popular treatments. But do they work?
- Finasteride
- Oral
- Topical
- Dutasteride
- Oral
- Topical
- Mesotherapy
- Minoxidil
- Oral
- Topical
- Ketoconazole
- Shampoo
- Topical
- Low-Level Laser Therapy
- Therapy
- Microneedling
- Therapy
- Platelet-Rich Plasma Therapy (PRP)
- Therapy
- Scalp Massages
- Therapy
More
IngredientsTop-selling ingredients, quantified.
- Saw Palmetto
- Redensyl
- Melatonin
- Caffeine
- Biotin
- Rosemary Oil
- Lilac Stem Cells
- Hydrolyzed Wheat Protein
- Sodium Lauryl Sulfate
More
ProductsThe truth about hair loss "best sellers".
- Minoxidil Tablets
Xyon Health
- Finasteride
Strut Health
- Hair Growth Supplements
Happy Head
- REVITA Tablets for Hair Growth Support
DS Laboratories
- FoliGROWTH Ultimate Hair Neutraceutical
Advanced Trichology
- Enhance Hair Density Serum
Fully Vital
- Topical Finasteride and Minoxidil
Xyon Health
- HairOmega Foaming Hair Growth Serum
DrFormulas
- Bio-Cleansing Shampoo
Revivogen MD
more
Key MetricsStandardized rubrics to evaluate all treatments.
- Evidence Quality
Is this treatment well studied?
- Regrowth Potential
How much regrowth can you expect?
- Long-Term Viability
Is this treatment safe & sustainable?
Free Research- Free Resources
Apps, tools, guides, freebies, & more.
- Topical Finasteride Calculator
- Interactive Guide: What Causes Hair Loss?
- Free Guide: Standardized Scalp Massages
- 7-Day Hair Loss Email Course
- Ingredients Database
- Interactive Guide: Hair Loss Disorders
- Treatment Guides
- Product Lab Tests: Purity & Potency
- Evidence Quality Masterclass
More
Articles100+ free articles.
-
Cannabidiol (CBD) Increases Hair Counts By 246%? Not So Fast.
-
Creatine: Does It Worsen Hair Loss? It Depends On The Hair Loss Type.
-
Can Progesterone Improve Hair Regrowth?
-
CRABP2: Can This Gene Predict Regrowth From Retinoids?
-
BTD: Can This Gene Predict Regrowth From Biotin?
-
COL1A1: Can This Gene Predict Regrowth From Collagen Support?
-
2dDR For Hair Loss: What Do We Know So Far About This Sugar?
-
CYP19A1: Can This Gene Predict Regrowth From Hormone Therapy?
PublicationsOur team’s peer-reviewed studies.
- Microneedling and Its Use in Hair Loss Disorders: A Systematic Review
- Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia
- Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results
- A Hypothetical Pathogenesis Model For Androgenic Alopecia:Clarifying The Dihydrotestosterone Paradox And Rate-Limiting Recovery Factors
Menu- AboutAbout
- Mission Statement
Education. Evidence. Regrowth.
- Team Members
PhD's, resarchers, & consumer advocates.
- Editorial Policy
Discover how we conduct our research.
- Contact
Have questions? Contact us.
- Before-Afters
 Built
BuiltWhat causes androgenic alopecia?
Enough with the strawman debates. Here are the strongest arguments for (and against) many popular theories of hair loss.
Viability
Hormones
The claim:Androgenic alopecia (AGA) is caused by the hormone dihydrotestosterone (DHT).
Viability
ArgumentCounterargumentRebuttalConsensusMechanistic
Mechanistic studies show that when AGA-prone hair follicles are exposed to DHT, dermal papilla cell clusters undergo apoptosis (cell death).
Observational
Observational data show that (1) men who lack the gene to make type II 5-alpha reductase – an enzyme that converts to free testosterone into DHT – do not go bald in adulthood, and (2) men castrated before puberty (who then reduce their exposure to male hormones by 95% throughout a lifetime) do not go bald in adulthood.
Interventional
Interventional studies show that (1) testosterone injections can stimulate AGA in castrated adults, and (2) DHT-reducing drugs – like finasteride and dutasteride – can slow, stop, or partially reverse AGA in 80-90% of men over a 1, 2, and 5-year period. This creates strong mechanistic, observational, and interventional plausibility that AGA is causally linked to male hormones and, in particular, the hormone DHT.
References
Diffuse female hair loss: are androgens necessary? Finasteride For Women: Effect & Safety Improvement in scalp hair growth in androgen-deficient women treated with testosterone: a questionnaire study Prospective cohort study on the effects and tolerability of flutamide in patients with female pattern hair loss Effects of flutamide on pituitary and adrenal responsiveness to corticotrophin releasing factor (CRF) Pediatric androgenetic alopecia: A review 5α-reductase activity in women with polycystic ovary syndrome: a systematic review and meta-analysisIf DHT is the main cause of AGA:
- How come people with complete androgen insensitivity syndrome (i.e., people who lack androgen receptors) can still develop pattern hair loss – despite having no androgenic activity?
- How come women with female pattern hair loss respond much less favorably to DHT-reducing drugs – like finasteride – than men?
- How come observational research suggests that testosterone injections in low-testosterone women with female pattern hair loss might improve hair growth outcomes?
- How come children – who have very little-to-no exposure to androgens – can still develop pattern hair loss?
- How come genetically identical twins – who carry the same genes and similar hormonal profiles – can go bald at different rates?
These facts suggest that factors outside of male hormones and DHT play a role in the development and progression of AGA.
References
Female pattern hair loss in complete androgen insensitivity syndrome Diffuse female hair loss: are androgens necessary? Finasteride For Women: Effect & Safety Improvement in scalp hair growth in androgen-deficient women treated with testosterone: a questionnaire study Androgenetic alopecia in children: report of 20 cases The contribution of endogenous and exogenous factors to female alopecia: a study of identical twins The contribution of endogenous and exogenous factors to male alopecia: a study of identical twins Eleven pairs of Japanese male twins suggest the role of epigenetic differences in androgenetic alopecia- ( In recent years, clinicians have begun to acknowledge that “male” androgenic alopecia and “female pattern hair loss” may constitute different types of genetic-mediated hair loss, and that these hair loss disorders perhaps never should’ve been grouped together. This notion comes from studies suggesting that (1) women with female pattern hair loss also often have low circulating androgen levels – with many women first showing signs of hair loss after menopause after their ovaries stop producing androgens, (2) women respond more poorly to finasteride and dutasteride than men, and (3) in low-testosterone women, testosterone replace therapy may subjectively improve hair parameters, rather than worsen them. Even still, androgens likely still play a significant role for many females with pattern hair loss. This is because androgen receptor-antagonizing drugs – like flutamide – have been shown to improve female pattern hair loss outcomes better than finasteride, and across all ages (even post-menopausal women). These studies also suggest sustained results with continued medication use, even 8+ years after initiating therapy. Interestingly, drugs like flutamide and bicalutamide reduce androgenic activity of all types (testosterone, DHT, etc.), and lower androgen production in both the ovaries and the adrenal glands. This suggests that perhaps other androgens or adrenal-produced hormones are involved in many cases of female pattern hair loss aside from DHT, and that androgens are still a factor.
- Female pattern hair loss tends to present in a diffuse pattern (i.e., Ludwig patterning), whereas male pattern hair loss tends to begin at the temples and crown (i.e., Norwood patterning). Interestingly, most pediatrics and adolescents with AGA have their hair loss present the same way as females: diffuse thinning, retention of the hairline, and hair loss toward the crown. These similarities in patterning further bolster the hypothesis that female pattern hair loss may not be the gender-mirroring of “male” AGA, and that children with AGA may have hair loss that shares a closer etiology to women with female pattern thinning than men with classical Norwood-Hamilton AGA presentations. For this reason, researchers speculate that AGA likely needs further subclassifications, and that non-androgenic factors may also be involved in many of these edge cases.
- Genetically identical twins carry the same genes and similar hormonal profiles. However, different lifestyle choices can increase the total androgenic activity of one twin versus another. Here are two examples: (1) testosterone use in one twin versus the other, and (2) metabolic syndrome in one twin versus the other – the latter of which would lead to increased insulin sensitivity and potentially increase 5-alpha reductase activity in certain tissues, thereby accelerating the balding process in the affected twin. Moreover, the progression of AGA tends to occur through hair follicle miniaturization, which advances through hair cycling. The studies on genetically identical twins correlated telogen effluvium-related dietary, lifestyle, and environmental choices with twins who were balding at faster rates – implicating that the differences in outcomes here could’ve merely been a function of stress-induced sheds that accelerated the balding process in one twin versus the other.
References
Diffuse female hair loss: are androgens necessary? Finasteride For Women: Effect & Safety Improvement in scalp hair growth in androgen-deficient women treated with testosterone: a questionnaire study Prospective cohort study on the effects and tolerability of flutamide in patients with female pattern hair loss Effects of flutamide on pituitary and adrenal responsiveness to corticotrophin releasing factor (CRF) Pediatric androgenetic alopecia: A review 5α-reductase activity in women with polycystic ovary syndrome: a systematic review and meta-analysisIn most men, AGA is likely caused by an interaction between genes and male hormones. However, AGA is a broad term that likely needs further subclassifications – as the development of pattern hair loss in children and women implicates non-androgenic pathways in some cases.
Genetic Predisposition
The claim:Androgenic alopecia (AGA) is caused by genetic predisposition.
Viability
ArgumentCounterargumentRebuttalConsensusObservational
Observational studies suggest varying rates of AGA across races. In men, genetic surveillance studies have found that AGA is associated with more than 220 genes involved in androgen metabolism, androgen receptor expression, and enzymes linked to the production of DHT. Observational studies in women suggest that AGA (or female pattern hair loss) is associated with genes that code for Wnt-β-catenin, TGF-α, TGF-β, Stat-3, Stat-1, PPARd, IGF-1, and other genes directly involved in hair cycling. Altogether, the data strongly suggest that AGA and female pattern hair loss are polygenic hair loss disorders with a direct relationship to genes tied to androgen activity and hair cycling. Finally, studies show that men lacking the gene that codes for type II 5-alpha reductase – which converts free testosterone into DHT – are protected from AGA throughout adulthood.
References
Male androgenetic alopecia Hunting the genes in male-pattern alopecia: how important are they, how close are we and what will they tell us? Genetic variation in the human androgen receptor gene is the major determinant of common early-onset androgenetic alopecia Understanding Pattern Hair Loss—Hair Biology Impacted by Genes, Androgens, Prostaglandins and Epigenetic FactorsBy itself, observational data cannot infer causality. Moreover, cross-sectional studies have demonstrated that genetically identical twins bald at different rates – with faster rates seen in twins who report poorer diets, higher rates of alcoholism, higher levels of stress, a higher number of divorces, and metabolic syndrome. This suggests that – in addition to genetics – dietary, lifestyle, and environmental choices may also drive the balding process, and that genes are not the end-all-be-all or the sole deterministic factor for balding.
References
The contribution of endogenous and exogenous factors to female alopecia: a study of identical twins The contribution of endogenous and exogenous factors to male alopecia: a study of identical twins Eleven pairs of Japanese male twins suggest the role of epigenetic differences in androgenetic alopeciaIn a vacuum, genes do not drive the entire balding process. However, when it comes to AGA and female pattern hair loss, the differences in balding rates between genetically identical twins is, in most cases, relatively small – with only minor differences in Norwood or Ludwig scores in most twins across a lifetime. For instance, one study found that just 8% of genetically identical twins expressed a “slight difference” late into adulthood. Finally, researchers do not argue that genes alone cause balding, but rather, that the interaction between genes and specific hormones – such as DHT – drive the majority of the balding process. This is supported through mechanistic, observational, and interventional research on both AGA and female pattern hair loss.
Mechanistic studies show that when cultured AGA-prone hair follicles are exposed to DHT, dermal papilla cell clusters undergo apoptosis (cell death). Observational data show that (1) men who lack the gene to make type II 5-alpha reductase – an enzyme that converts to free testosterone into DHT – do not go bald in adulthood, and (2) men castrated before puberty (thereby lowering their lifetime androgen exposure by ~95%) do not go bald in adulthood. Interventional data show that (1) AGA can be stimulated in castrated male adults who injected with testosterone, and (2) lowering type II 5-alpha DHT with drugs like finasteride and dutasteride can slow, stop, or partially reverse hair loss for 80-90% of AGA-affected men over 1, 2, and 5-year timelines.
Altogether, the current landscape of observational and interventional data show a significantly larger effect size supporting an interaction between genes and hormones in the progression of AGA versus all other explored factors.References
Intrapair differences of physical aging and longevity in identical twins Male androgenetic alopecia Induction of transforming growth factor-beta 1 by androgen is mediated by reactive oxygen species in hair follicle dermal papilla cells 5-Alpha-Reductase Deficiency Male hormone stimulation is prerequisite and an incitant in common baldness Efficacy and safety of finasteride therapy for androgenetic alopecia: a systematic reviewAGA is predominantly driven by an interaction between genes and hormones. While dietary, lifestyle, and environmental factors may accelerate AGA, gene-hormonal interactions still likely drive the majority of the balding process.
Scalp Tension
The claim:Androgenic alopecia (AGA) is driven by scalp tension generated from chronic contraction of the muscles anchored to the galea aponeurotica. These muscles pull the scalp skin taut – like a drum – and initiate an inflammatory cascade that, along with androgens, leads to hair follicle miniaturization and the progression of AGA.
Viability
ArgumentCounterargumentRebuttalConsensusMechanistic
Two-dimensional von Mises models show that when the frontalis and occipitalis muscles of the scalp are contracted, these contractions create a pattern of tension that near-perfectly aligns with the balding process. In hypoxic (i.e., low-oxygen) environments, cell culture studies suggest that free testosterone may preferentially convert not but estradiol, but to DHT – the hormone causally associated with AGA. This provides biological plausibility that scalp tension may compress the microvasculature in the skin above the galea aponeurotica, thereby decreasing oxygen, increasing DHT, and initiating the balding process in genetically predisposed hair follicles.
Observational
Observational studies on balding scalps routinely show the presence of increased androgenic activity, decreased oxygen levels, increased inflammatory signaling proteins such as TGF-B1, and increased scarring. These same biological and histological markers are present in biopsies of other tissues affected by disease states involving chronic pressure and/or tissue tension: benign prostatic hyperplasia, Dupuytren’s contracture, and the retracted eyelids of Grave’s Disease patients.
Interventional
Interventional data across 5+ studies on botulinum toxin A injections (Botox®) into the scalp muscles of AGA-affected men and women show a 75-80% response rate and an average 18-21% increase in terminal counts over 6 months.
This mechanistic, observational, and interventional data suggest that scalp tension is involved in the balding process, and that relieving this tension by way of botulinum toxin A injections into scalp muscles may slow, stop, and/or partially reverse AGA.
References
A hypothetical pathogenesis model for androgenic alopecia: clarifying the dihydrotestosterone paradox and rate-limiting recovery factors Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review Study of the Effectiveness and Safety of Botulinum Toxin Type A for The Treatment of Androgenetic Alopecia in Men- If the scalp environment is causally linked to AGA, how come hairs transplanted to balding areas survive long after other hairs in that area disappear?
- How is it possible that the contraction of the scalp perimeter muscles can reduce blood supply to the top of the scalp when the main arteries supplying blood to these regions rest above those muscles?
- Observational studies show that hair follicle miniaturization – the defining characteristic of AGA – is a step-process that occurs through hair shedding and that hair cycling is the primary driver of reduced blood supply in balding regions, suggesting low oxygen is a consequence of the balding process rather than a cause.
- The interventional studies on botox did not have control groups and thus the effects on hair growth cannot be discerned beyond seasonal hair cycling.
- One research group demonstrated that the effects of botox on hair growth are likely from reductions to TGF-B1, not scalp tension.
- If tension generated from contracted scalp muscles causes AGA, how come AGA can also occur in other areas of the scalp, and not just above the galea aponeurotica?
References
Hair Transplant Longevity Parts of the Scalp: A Guide to the Anatomy, Mechanics, and New Treatment Possibilities Conflicting Reports Regarding the Histopathological Features of Androgenic Alopecia Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review Study of the Effectiveness and Safety of Botulinum Toxin Type A for The Treatment of Androgenetic Alopecia in Men The effect of intradermal botulinum toxin on androgenetic alopecia and its possible mechanism Occipital involvement in female pattern hair loss: histopathological evidences- Despite popular belief, there is no long-term data on the survivability of hair transplants in AGA. In fact, the one study with > 25 participants examining their survivability four years post-transplant concluded that > 90% of participants showed a loss in transplanted hairs, regardless of their use of FDA-approved drugs. More recently, leaders and researchers involved in the International Society for Hair Restoration finally acknowledged that their transplant patients are coming back 10+ years later showing evidence of the loss of the hair, and that the original hypothesis from Orentreich of transplant “donor dominance” might not actually be 100% correct.
- While many arteries of the scalp reside above the scalp perimeter muscles, there are several arteries – many of which predominate two-thirds of the blood supply to the front of the scalp – that reside underneath, in between, and directly intersect these muscles – meaning that their chronic contraction would have an impact on their blood supply. Moreover, observational studies on those with chronic tension headaches demonstrate that during periods of muscle contraction, the arteries above those muscles also dilate by 50%, thus leading to a 75% reduction in blood supply. This indicates that the contraction of these muscles might actually also affect arteries above them, even if some arteries don’t directly run through the muscles. Finally, the top of the scalp is a fixed space, and the contraction of these muscles pulls the skin above the galea aponeurotica tight, thereby compressing the microvasculature in the subcutaneous layers that reside directly below AGA-prone hair follicles. This chronic tension-induced compression would reduce blood supply in these regions, regardless of reductions to blood supply in the surrounding arteries.
- It can be simultaneously true that reductions to blood supply are both a consequence of hair follicle miniaturization and a cause of hair follicle miniaturization; these ideas are not mutually exclusive.
- It is true that the studies on botox did not have control groups, which makes it harder to discern the outcomes from any six-month study versus hair cycle seasonality. However, fluctuations to the hair cycle typically only account for 5-10% changes to hair counts throughout the year. All intramuscular botox studies demonstrate average hair count increases in the range of 18-21%, which far exceed seasonal fluctuations to the hair cycle. Therefore, even without a control group, the results warrant further investigation.
- One research group did demonstrate in vitro that human hair follicle cell cultures exposed to botulinum toxin A showed reduced activity of TGF-B1 – a negative growth factor for hair growth and a treatment target for AGA. Those same researchers also conducted a study on injections of Botox® conducted directly into balding regions, and showed a 5% increase in terminal hair counts over 4 months of use. In the Discussion section of their paper, they speculated that in the intramuscular Botox®-AGA studies, the medication might’ve diffused from the muscles, traveled through the bloodstream to the top of the scalp, and then produced a reduction in TGF-B1 – thus potentiating its hair growth-promoting effects. This speculation defies all pharmacokinetic understandings of botulinum toxin A – particularly in relation to its maximal spread. Multiple anhidrosis studies of Botox® injected into the frontalis muscles show that the maximal effects of the medication spread 15-30 millimeters vertically from the scalp injection sites. In the intramuscular Botox®-AGA studies, hair count measurements were made at the crown – roughly 150 millimeters away from the closest intramuscular injection site. Therefore, in order for this speculated mechanism to apply to the effects in the intramuscular Botox®-AGA studies, Botox® injected intramuscularly would’ve needed to escape the muscles, travel 10-folder further than its maximal effect spread to the top of the scalp, and then arbitrarily settle in these scalp tissues without continuing to dilute or travel systemically. Moreover, the only cases where Botox® has been shown to rupture from muscles in appreciable volumes and travel systemically in appreciable volumes is in animal studies that injected a high volume of saline and low amount of Botox®. These methodologies are not reflected in any of the intramuscular Botox®-AGA studies, and thus significant systemic spread is unlikely. When the authors of the intradermal study were asked over email if any of this changed their speculations of Botox® diffusion, they never replied. Therefore, the totality of pharmacokinetic data, interventional data, and expert testimony suggest that Botox® injected into scalp muscles cannot diffuse and/or resettle in a such a way suggested by the authors of the intradermal Botox®-AGA study, and that the mechanism of action for intramuscular Botox® likely has to do with the muscles themselves, rather than the diffusion of Botox®.
- It is true that AGA can also develop in areas outside of the galea aponeurotica, particularly in later stages of AGA and in females with pattern hair loss. While it is possible that hair loss in these regions might be from other causes and may not be entirely androgenic in nature, it’s more likely that this is an indication that scalp tension is not the primary driver of AGA, but rather, an accelerator of the condition.
References
Hair Transplant Longevity Parts of the Scalp: A Guide to the Anatomy, Mechanics, and New Treatment Possibilities Seasonal changes in human hair growth The Change of Plane of the Supratrochlear and Supraorbital Arteries in the Forehead—An Ultrasound-Based Investigation Comparison of the spread of three botulinum toxin type A preparations Diffusion of Two Botulinum Toxins Type A on the Forehead: Double-Blinded, Randomized, Controlled Study Botox produces functional weakness in non-injected muscles adjacent to the target muscle Re: Botox produces functional weakness in non-injected muscles adjacent to the target muscleScalp tension may accelerate AGA, and perhaps in 75% of men with standard Norwood-Hamilton AGA presentations. However, consensus is that scalp tension is not the root cause of AGA, nor is it a possible accelerator of all subclassifications of AGA. More robust studies exploring intramuscular botulinum toxin A injections are required to elucidate the medication’s true interventional power and mechanism(s).
Diet, Lifestyle, & Environment
The claim:Androgenic alopecia (AGA) is driven by scalp tension generated from chronic contraction of the muscles anchored to the galea aponeurotica. These muscles pull the scalp skin taut – like a drum – and initiate an inflammatory cascade that, along with androgens, leads to hair follicle miniaturization and the progression of AGA.
Viability
ArgumentCounterargumentRebuttalConsensusMechanistic
Cell culture studies suggest that stress hormones, vitamins, and nutrients all play a role in the regulation of human hair cycling. Cell culture studies show that insulin resistance in certain skin tissues may also increase 5-alpha reductase activity, and thereby levels of dihydrotestosterone (DHT) – the hormone causally associated with AGA.
Observational
Three separate studies have shown that genetically identical twin studies can go bald at different rates – with the faster-balding twins reporting a history of lower-quality diets, higher rates of alcoholism, higher levels of stress, a higher number of divorces, and a higher incidence of metabolic syndrome. Age-controlled studies show a faster progression of AGA in smokers versus non-smokers. Observational studies also show significant correlations between diets containing sugar-sweetened beverages and a faster progression of AGA.
Interventional
A spectrum of dietary, lifestyle, and environmental choices causally influence our levels of systemic inflammation, hormone production, and overall health. Dietary, lifestyle, and environmental choices are not only capable of normalizing insulin sensitivity in type II diabetics, but also lowering inflammation and reducing androgen activity.
Therefore, it is reasonable to assume that diet, lifestyle, and environment may causally drive the balding process, and that these same levers might be used to also improve outcomes for AGA.
References
Diets high in selenium and isoflavones decrease androgen-regulated gene expression in healthy rat dorsolateral prostate Dietary zinc deficiency alters 5 alpha-reduction and aromatization of testosterone and androgen and estrogen receptors in rat liver Role of insulin and insulin resistance in androgen excess disorders The contribution of endogenous and exogenous factors to female alopecia: a study of identical twins The contribution of endogenous and exogenous factors to male alopecia: a study of identical twins Eleven pairs of Japanese male twins suggest the role of epigenetic differences in androgenetic alopecia Role of Smoking in Androgenetic Alopecia: A Systematic Review The Association between Sugar-Sweetened Beverages and Male Pattern Hair Loss in Young Men The Role of Diet on Insulin Sensitivity Manipulation of Dietary Intake on Changes in Circulating Testosterone ConcentrationsNo interventional studies exist to determine if modifications to diet, lifestyle, or environment have any impact on AGA. While there is mechanistic and interventional evidence showing certain dietary, lifestyle, and environmental choices can reduce inflammation and normalize androgen activity, we cannot assume that these effects will automatically translate to improvements in hair loss disorders. For instance, it often takes more than a sustained 50% reduction in scalp DHT to produce improvements to AGA in men. While some aspects of living can be changed to normalize DHT levels, there are no studies demonstrating that any dietary, lifestyle, or environmental intervention can consistently or sustainably lower DHT levels by 70% in the scalp – let alone the serum.
Finally, while the studies on genetically identical twins are interesting and warrant further investigation, these findings generally demonstrate relatively small differences in balding rates across twins, and throughout a lifetime – even despite reportedly large differences in diet, lifestyle, and environment. This suggests that while these factors might play a role in AGA progression, their overall impact is still small, and cannot account for the majority of balding between each set of twins. One study found that only 8% of identical twins showed a “slight difference” in balding late into middle age. In fact, the difference in balding rates across twins is most likely explainable through additional bouts of telogen effluvium for the faster-balding twin, which would accelerate the balding process by accelerating the hair cycle and creating more opportunities for hair follicle miniaturization in that twin. Therefore, diet, lifestyle, and environment are most likely secondary causes of AGA progression, but not the root cause of AGA itself.References
Clinical dose ranging studies with finasteride, a type 2 5alpha-reductase inhibitor, in men with male pattern hair loss Manipulation of Dietary Intake on Changes in Circulating Testosterone Concentrations Intrapair differences of physical aging and longevity in identical twins The contribution of endogenous and exogenous factors to female alopecia: a study of identical twins The contribution of endogenous and exogenous factors to male alopecia: a study of identical twinsWhile interventional studies on diet, lifestyle, and hair loss do not exist, the absence of evidence does not imply evidence againstsomething. Prospective studies are warranted to tease out the exact effects of diet, lifestyle, and environment on AGA development.
Certain dietary, lifestyle, and environmental factors may accelerate AGA, but their overall impact is still small relative to the involvement of genes and androgens.
Blood Flow
The claim:Reduced blood flow causes androgenic alopecia (AGA).
Viability
ArgumentCounterargumentRebuttalConsensusMechanistic
Mouse model data and scalp biopsies in humans show that drugs that promote hair growth – like minoxidil – also promote the fenestration of blood vessels supporting hair follicles (i.e., angiogenesis in balding regions).
Observational
Balding scalp regions show 40% reductions to transcutaneous oxygen levels versus controls, and more than a 2.6-fold decrease in subcutaneous blood flow versus controls.
Interventional
Minoxidil – a potassium ion channel opener that causes vasodilation in microvascular networks – regrows hair in men and women with AGA when applied topically and taken orally.
References
Minoxidil use in dermatology, side effects and recent patents The induction by topical minoxidil of increased fenestration in the perifollicular capillary wall Subcutaneous Blood Flow in Early Male Pattern Baldness Transcutaneous PO2 of the scalp in male pattern baldness: a new piece to the puzzle Topical Minoxidil: Systematic Review and Meta-Analysis of Its Efficacy in Androgenetic AlopeciaLow blood flow is likely a consequence of AGA, rather than a root cause.
- If low blood flow were the primary cause of AGA, all blood pressure-lowering medications would improve hair growth. However, this effect isn’t seen with all blood pressure-lowering medications. It is only seen with minoxidil.
- Minoxidil is suspected to work through other mechanisms beyond vasodilation – such as through modifications to anti-inflammatory substances like prostaglandins. Observational studies show that prostaglandins are elevated in balding regions, and in mouse models, that reducing these prostaglandins improves hair lengthening. For these reasons, minoxidil’s other mechanisms of action are likely responsible for its hair growth-promoting effects.
- AGA progresses through hair follicle miniaturization, which occurs as a single step-process in between hair cycles – when an old hair sheds and a new hair regenerates to take its place. As these newly miniaturized hairs begin to form, smaller microvascular networks grow in from the subcutaneous tissue to support them, and not the other way around. This suggests that reductions to blood flow occur as a result of hair follicle miniaturization, rather than a cause of hair follicle miniaturization.
- This is incorrect. Other vasodilation medications do improve hair growth – such as diazoxide and pinacidil. Both of these medications, like minoxidil, improve vasodilation by targeting potassium ion channels. This is important, because anti-hypertensive agents have (1) different magnitudes of effect, and (2) different effects across the body – in particular, the microvasculature in the subcutaneous layers of skin below AGA-prone hair follicles. For instance, peppermint oil happens to cause vasodilation in the vascular endothelium but vasoconstriction in vascular smooth muscle tissues – all because it leverages different mechanisms than minoxidil, despite having similar effects across some regions of the body. Therefore, it is scientifically disingenuous to group together all anti-hypertensive and/or vasodilators as evidence that minoxidil doesn’t work by increasing blood flow, especially when multiple anti-hypertensive agents that improve vasodilation by targeting potassium ion channels all seem to regrow hair just as minoxidil does. Depending on the medication, dosing schedule, delivery method, and regions of application – anti-hypertensive compounds can have a range of blood flow-related effects across organ sites, arteries, veins, and microvascular networks – some of which are paradoxical.
- While minoxidil was once suspected to potentially improve AGA through its modification of prostaglandins, further research suggests this hypothesis is not as strong as once suspected. While mechanistic and observational data show that changes to prostaglandin activity are associated with AGA progression, studies testing “causality” failed to show effects. For instance, an interventional study on setipiprant – a prostaglandin D2 inhibitor – found that the drug had no effect versus placebo at improving outcomes of AGA. Other studies testing prostaglandin analogues or modifiers – such as latanoprost and bimatoprost – are poorly designed, show low response rates, and small effect sizes on hair parameters. Finally, future studies conducted by Garza et al. (and other research groups) have found conflicting results regarding the presence of prostaglandins throughout the progression of AGA – with some studies showing elevated pro-inflammatory and anti-inflammatory prostaglandin levels only during specific stages of the hair cycle or specific stages of AGA progression. Altogether, these findings have significantly dampened excitement surrounding prostaglandins as a potential treatment target for AGA, and have only served to bolster the possibility that minoxidil might actually work through other mechanisms.
- It can be simultaneously true that low blood flow is a consequence of hair follicle miniaturization in AGA, and also an effective secondary treatment target for its improvement. After all, 5-year studies on minoxidil demonstrate hair count improvements that remain above baseline, albeit that begin waning after year one. While the data on DHT-reducing drugs – like finasteride and dutasteride – demonstrate superior quality of evidence for long-term efficacy, it is also possible that minoxidil and other potassium ion channel-opening agents can improve hair growth, even if they don’t fully stop miniaturization mediated by dihydrotestosterone (DHT).
References
Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil Hypertrichosis Induced by Diazoxide in Idiopathic Hypoglycemia of Infancy Clinical Pharmacology of Pinacidil, A Prototype for Drugs That Affect Potassium Channels Potassium Channel Conductance: A Mechanism Affecting Hair Growth both In Vitro and In Vivo Minoxidil For AGA: Debating 3 Suspected Mechanisms Of Action Setipiprant for Androgenetic Alopecia in Males: Results from a Randomized, Double-Blind, Placebo-Controlled Phase 2a TrialReductions to blood flow are likely secondary factors involved in AGA, rather than the root cause of AGA. However, multiple anti-hypertensive agents that target to open potassium ion channels have demonstrated some efficacy in AGA, and so this treatment target shouldn’t be neglected entirely.
Prostaglandins
The claim:Prostaglandins – which are inflammatory fatty acid derivatives – cause androgenic alopecia (AGA).
Viability
ArgumentCounterargumentRebuttalConsensusMechanistic
In 2012, a renowned research team from the University of Pennsylvania demonstrated that,in mouse models, prostaglandin D2 inhibited hair lengthening.[15][16]
Moreover, in vitro studies suggest that minoxidil appears to enhance the activity of prostaglandins associated with the growth phase of the hair cycle, and perhaps dampen prostaglandins associated with hair loss.[17]Observational
Biopsy-related studies suggest that prostaglandin D2 (PGD2) and prostaglandin J2 (PGJ2) are elevated in balding regions.
Interventional
Clinical studies show that minoxidil – a drug that modifies prostaglandins – routinely regrows hair in men and women with AGA. Moreover, prostaglandin analogues and modulators like bimatoprost and latanoprost have also demonstrated mild hair growth-promoting effects.
Altogether, this data suggest that prostaglandins are causally involved in the balding process.
While prostaglandins were once suspected to be causally involved in the balding process, the initial mechanistic and observational studies establishing this link have not held up in robust interventional studies. For instance, an interventional study on setipiprant – a prostaglandin D2 inhibitor – found that the drug had no effect versus placebo at improving outcomes of AGA. Other studies testing prostaglandin analogues or modifiers – such as latanoprost and bimatoprost – are poorly designed, show low response rates, and small effect sizes on hair parameters. Finally, future studies conducted by Garza et al. (and other research groups) have found conflicting results regarding the presence of prostaglandins throughout the progression of AGA – with some studies showing elevated pro-inflammatory and anti-inflammatory prostaglandin levels only during specific stages of the hair cycle or specific stages of AGA progression. Finally, minoxidil has several suspected mechanisms – many of which do not involve prostaglandin modulation. Altogether, these findings have significantly dampened excitement surrounding prostaglandins as a potential treatment target for AGA.
References
Minoxidil For AGA: Debating 3 Suspected Mechanisms Of Action Intrapair differences of physical aging and longevity in identical twins Setipiprant for Androgenetic Alopecia in Males: Results from a Randomized, Double-Blind, Placebo-Controlled Phase 2a Trial Latanoprost for AGA: Evidence InvestigationIt is irresponsible to group together all prostaglandin analogues tested for the treatment of AGA – setipiprant, latanoprost, bimatoprost, minoxidil, and more – and assume that their mild effects do not infer causality or that all of these prostaglandin analogues have effectively ruled out the involvement of prostaglandins as a treatment target for AGA. Keep in mind that different drug doses, delivery methods, frequencies of use, and trial durations could’ve changed the results of any one of these studies. Also keep in mind that there are more prostaglandin analogues to be explored, and that research on this topic is still in its infancy.
If prostaglandin activity is causally linked to AGA, the studies (so far) suggest it is a smaller factor overall, and should remain a secondary rather than primary treatment target.
Prolactin Receptors
The claim:Elevated prolactin is the cause of AGA.
Viability
ArgumentCounterargumentRebuttalConsensusMechanistic
Mouse models show that prolactin plays a regulatory role in hair cycling, with prolactin suppression causing improved hair growth.
Observational
Observational studies suggest that hyperprolactinemia (i.e., high prolactin levels) are present in some women with female pattern hair loss.
Interventional
In stump-tailed macaques affected by AGA, HMI-115 – a drug that lowers the activity of prolactin by producing antibodies to the prolactin receptor – was shown to nearly double terminal hair counts over a period of 6 months.
Altogether, this suggests that prolactin might be causally associated with AGA, and that targeting to reduce prolactin (or prolactin receptors) might improve AGA outcomes.
While there is mechanistic research in mice suggesting that hair cycling is, in part, negatively regulated by prolactin, these findings don’t always stand up to observations in human studies. For reference, prolactin levels in women are highest during pregnancy, but female hair density during pregnancy improves. Moreover, association studies have found no statistically significant differences in serum prolactin between women with female pattern hair loss versus controls. Finally, interventional studies on animals rarely translate to humans. While the results of the study on stump-tailed macaques are intriguing, we need to keep in mind that this data is published in a patent filing, not a peer-reviewed journal, with the authors having a significant conflict of interest in filing positive results to garner interest in their patented drug.
While it’s true that animal models don’t always translate to human studies, stump-tailed macaques are unique in that they’re one of the only other species to also suffer from androgenic alopecia. Therefore, people should remain excited about the possibility that prolactin might be a novel treatment target for AGA, and that prolactin receptor antibodies might improve the condition.
There is currently not enough evidence to determine if prolactin is causally associated with AGA, or if lower prolactin will improve AGA outcomes.
Want to contribute?
This guide is built by our research team, refined by our community, and revised regularly.
Want to request a new hair loss factor? Or share a study that might challenge the validity of any argument, counterargument, rebuttal, or consensus? Contact us. If what you share helps strengthen the document, we`ll update is as soon as we can.
"... Can’t thank @Rob (PHH) and @sanderson17 enough for allowing me to understand a bit what was going on with me and why all these [things were] happening ... "
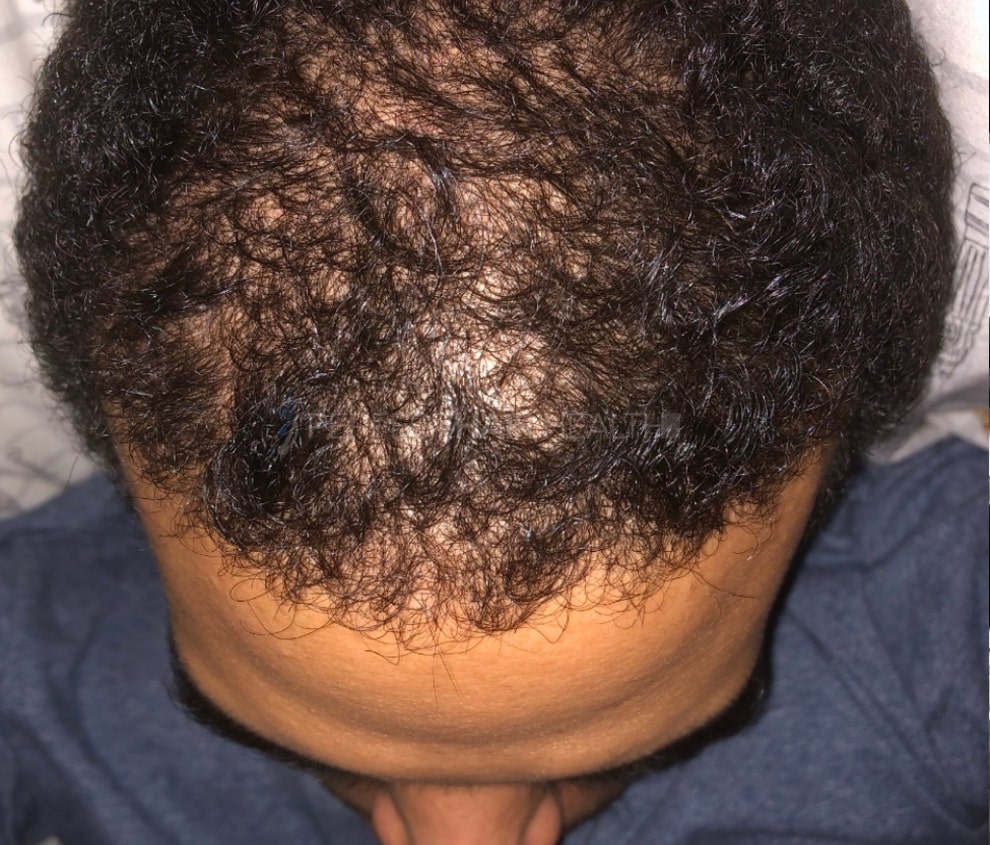
 — RDB, 35, New York, U.S.A.
— RDB, 35, New York, U.S.A."... There is a lot improvement that I am seeing and my scalp feel alive nowadays... Thanks everyone. "
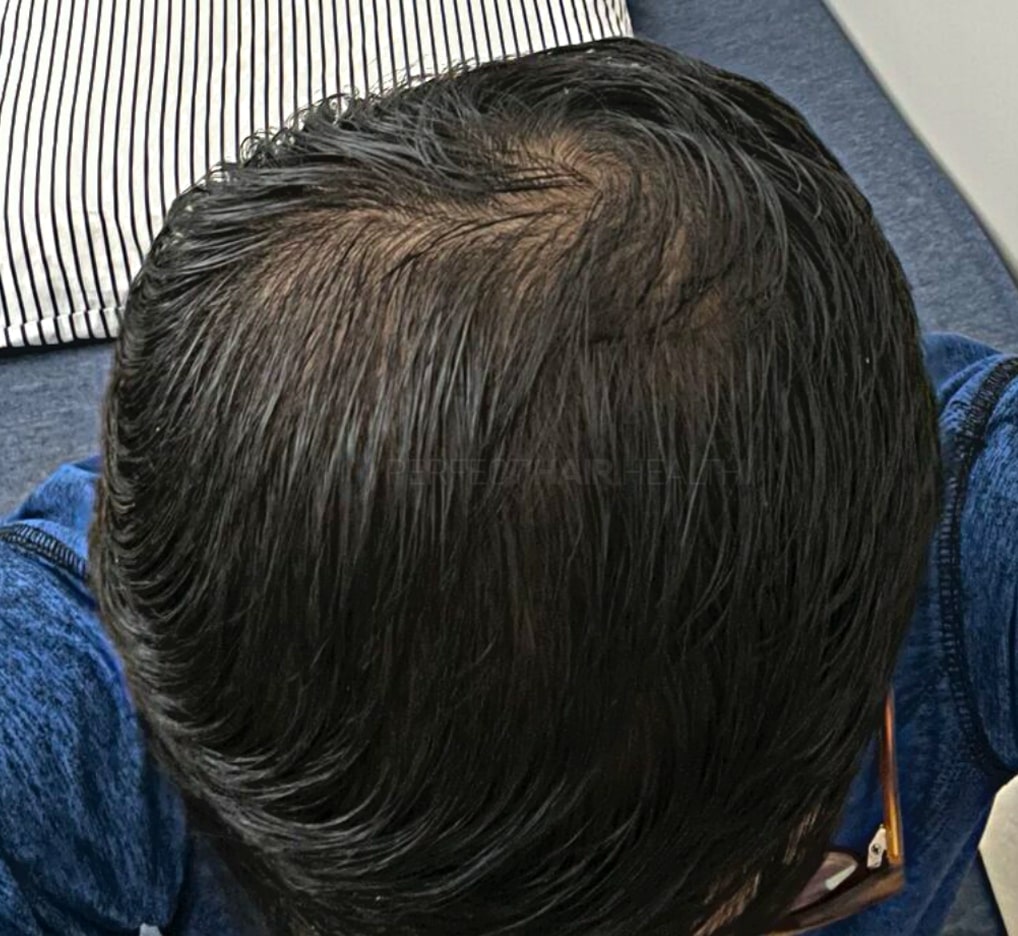 — Aayush, 20’s, Boston, MA
— Aayush, 20’s, Boston, MA"... I can say that my hair volume/thickness is about 30% more than it was when I first started."
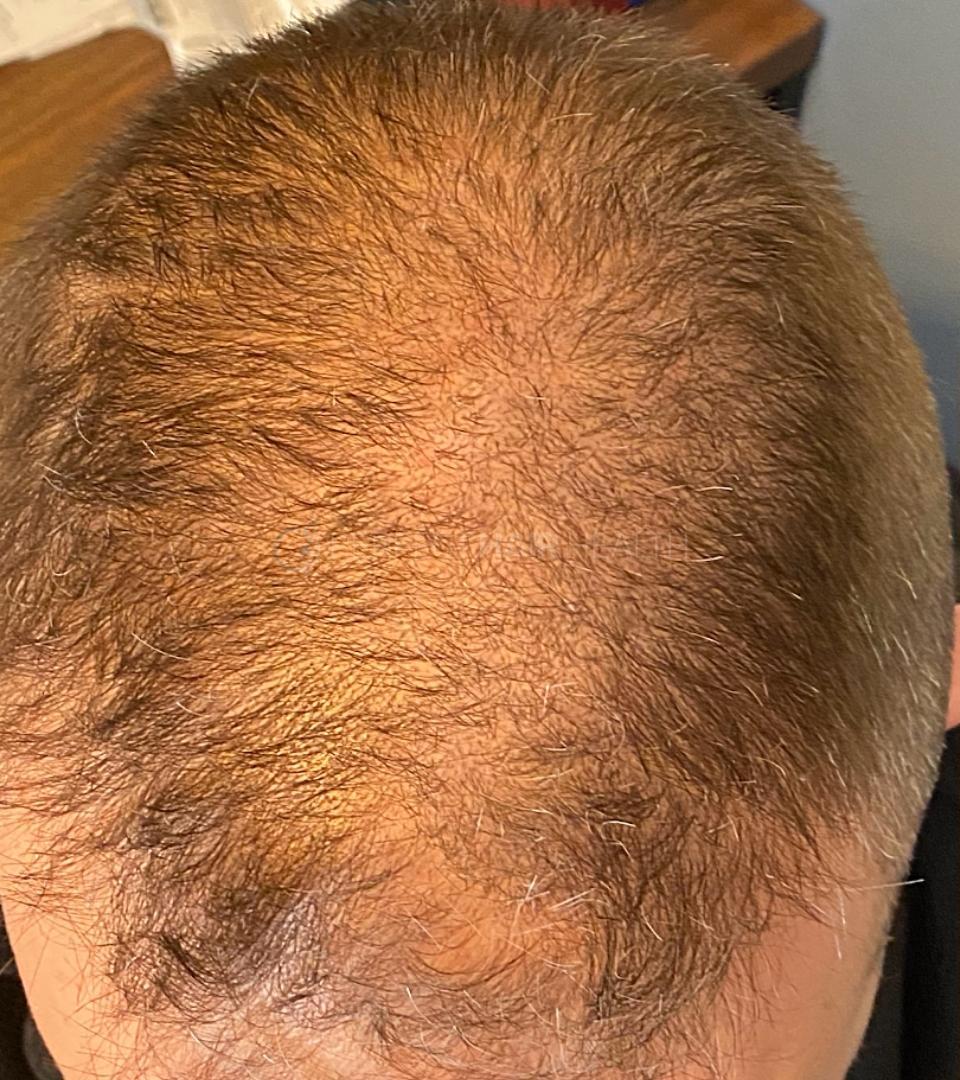
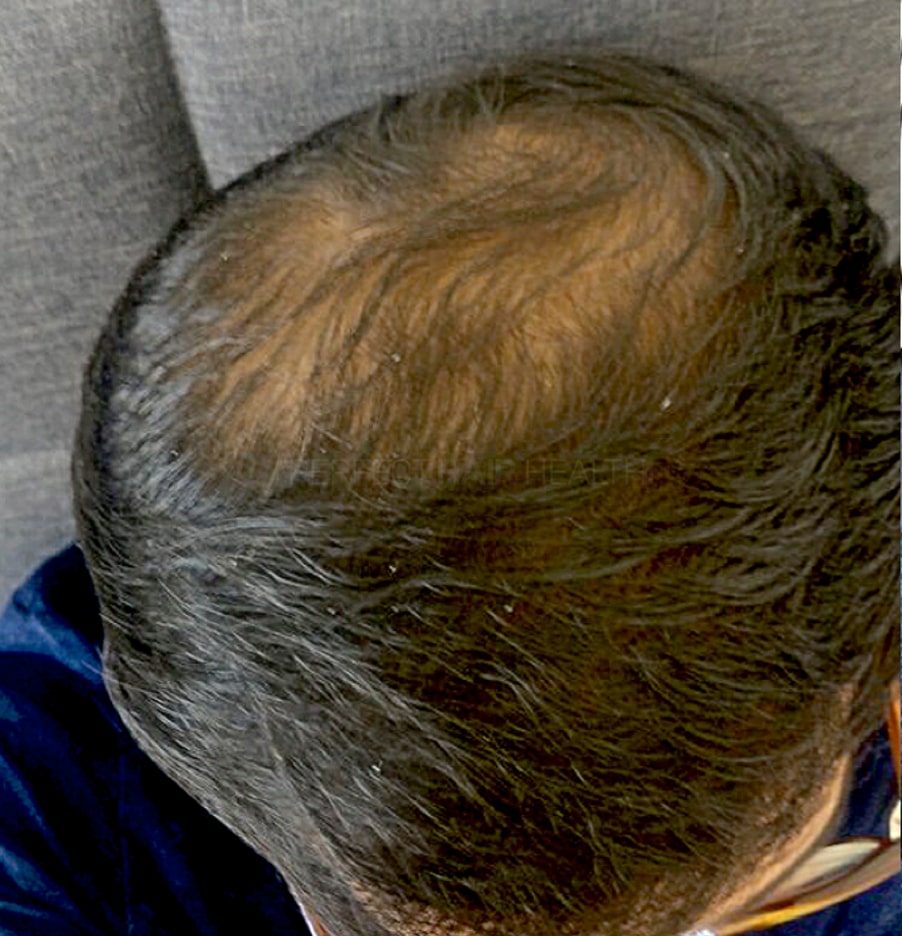
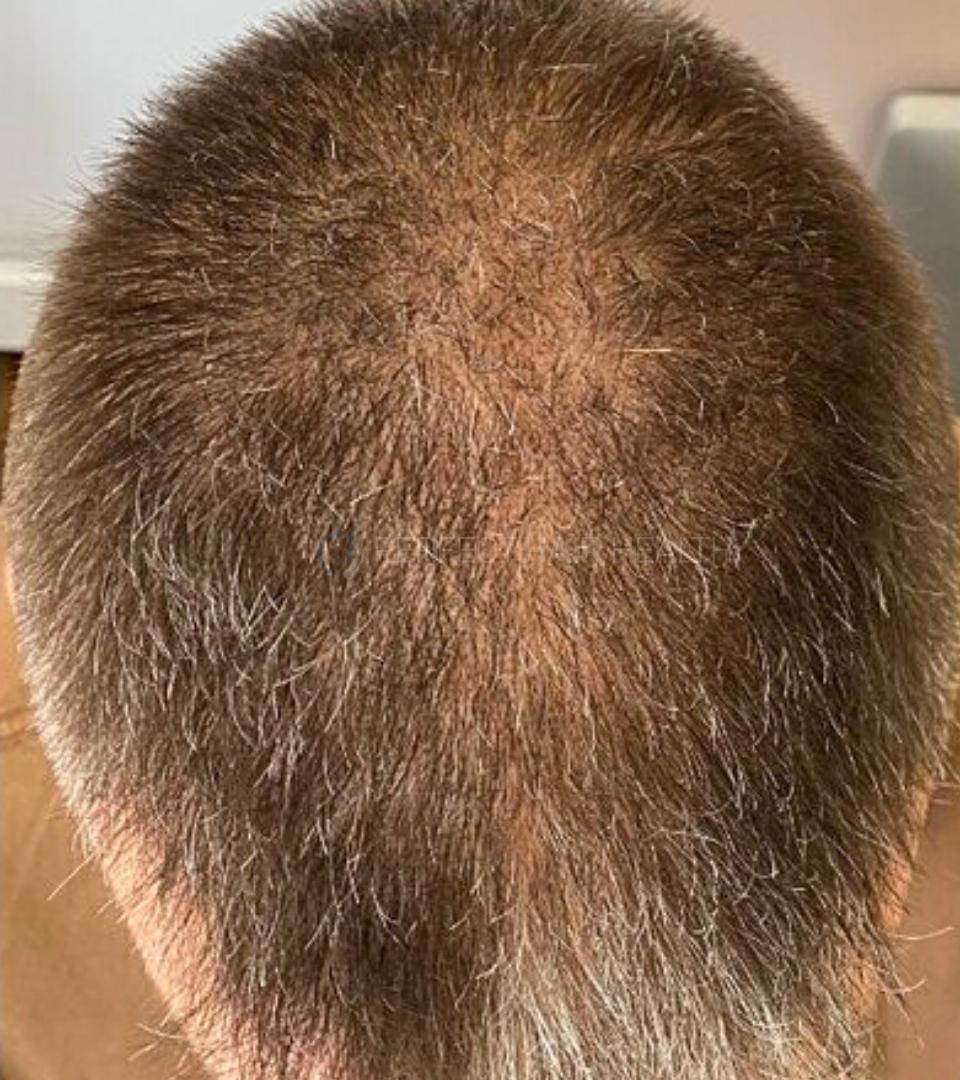 — Douglas, 50’s, Montréal, Canada
— Douglas, 50’s, Montréal, CanadaWant help with your hair regrowth journey?
Get personalized support, product recommendations, video calls, and more from our researchers, trichologists, and PhD's dedicated to getting you the best possible outcomes.
Join Now - Mission Statement